By Jara Pérez-Jiménez
Proanthocyanidins (PAs) are a class of dietary phenolic compounds, present in foods or beverages such as berries, legumes, wine, cocoa and derived products or nuts. They are important contributors to total polyphenol content in some food items and these values are even higher when taken into account that a fraction of PAs, the non-extractable proanthocyanidins, with high molecular weight, are not usually considered in the estimations of polyphenol content or intake [1]. PAs include dozens of structures, since they go from dimers to long polymeric compounds, constituted by several substituents. Once ingested, only dimers are directly absorbed, while the other PAs may be transformed by colonic microbiota leading to bioactive metabolites, while a fraction will be excreted without being transformed [2]. Microbial modifications originate several metabolites from the original structures, a number that may be increased after absorption, since they can be conjugated by hepatic enzymes to different derivatives. For this reason, the analysis of PA derived metabolites as marker of their intake is a difficult task, requiring equipment and expertise that may not be available in any nutrition laboratory. On the other hand, there is an increasing interest for identifying intake biomarkers, due to the limitations associated to food recalls.
Based on these aspects, in our laboratory we decided to explore whether the determination of faecal proanthocyanidins by a spectrophotometric method was valid as a biomarker of total PA intake. Of course, this measurement is much less specific than HPLC-MS analysis of derived metabolites but, at the same time, it is easy to perform, so it might be an alternative for some laboratories. And although, obviously, faecal PAs have not been absorbed, it is known that there is an association between the excretion of PA derived metabolites and their intake [3], so it could be the same for total PAs. Besides, due to the increasing interest in microbiota, it is becoming common to collect faeces in studies with polyphenols, since microbiota profile and polyphenol metabolites are associated in these samples [4].
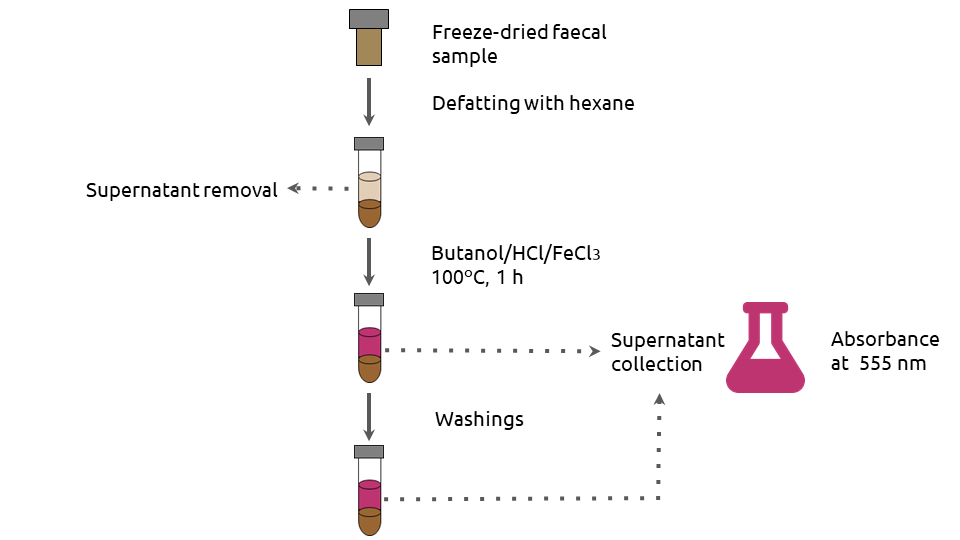
So we adapted Porter’s method [5], which was developed for evaluating PA content in plant material, in order to apply it in faeces, implying for instance, a previous defatting step for removing interferences. Figure 1 provides an overview of our final protocol. An interesting point is that, with this method, both extractable PAs (EPAs) and NEPAs are measured.
After modifying the method for its application in faecal samples, and in collaboration with researchers from the University of Barcelona and the University of California Davis, we measured total PA in faeces from pigs supplemented with a grape seed extract, rich in PAs. We found that when there was no supplementation there was no response in the method, what implied that we did not have false positive values. At the same time, there was a clear increment in faecal PAs evaluated by this method when the animals started to consume the extract. This increment was also present one day after stopping the treatment, due to the delayed metabolic transformation of some PAs and in subsequent days we obtained no response. Also, these values were significantly correlated with previous determinations in the same samples of some PA metabolites, (+)-catechin, (-)-epicatechin and syringic acid, as well as with the determination of PA dimers, trimers, tetramers and pentamers [4]. So it seemed that, in the context of a supplementation with a high PA dose, the determination of total faecal PAs may be a valid intake biomarker.
Then, we decided to move to a situation of habitual PA intake, without supplementation. We did this in collaboration with other researchers from our centre and Madrid Complutense University and we evaluated total faecal PAs in samples from 100 subjects participating in an observational study. Diet information, by three 24 h dietary recalls was collected but not on the previous day for providing the faecal sample. We decided to use this approach because, for some intake biomarkers, it has been shown that they are able to reflect the usual intake of a particular compound, even when it is not consumed immediately before providing the sample, which is very practical in studies with big population groups. However, in our case we did not detect any association between PA intake in the food recalls and total faecal PAs (by the way, in many samples we did not detect PAs, what allowed to confirm that the method was specific and it did not always provide positive values). We suggested some reasons for the fact that this approach (collection of biological samples and diet information not at the same time) had allowed to find intake biomarkers for some foods, such as coffee [6], or compounds, as total polyphenols [7], but was not useful here: in those cases, there is either a clear difference between consumers and non-consumers, or a wide distribution of those compounds in many different foods, leading to a sustained daily consumption. Nevertheless, in the case of PAs the situation is different: they appear in many foods, so it is not such a case of consumers/non-consumers. Also, they are not as widespread as total polyphenols (for instance, they are absent in many fruits) and the concentration range in foods is very different, so there may be higher differences in the day-to-day consumption than in the case of total polyphenols.
So, coming back to our initial question on whether total faecal PAs may be considered as intake biomarker for these compounds, our results showed that this is valid in acute supplementation with high doses, but it is not as marker of usual intake when faecal samples are randomly collected. Nevertheless, we still have to evaluate whether, in a joint collection of faeces and dietary information, the levels of faecal proanthocyanidins would reflect the habitual intake of these dietary bioactive compounds.

Jara Pérez-Jiménez completed holds a PhD in Food Science and Technology. She has worked in several research centres and universities in Spain and France focused on the study of food bioactive compounds, in particular polyphenols, using a multidisciplinary approach (studies on food composition, development of an online database, preclinical and clinical trials, observational studies). Currently, she is a Tenured Scientist at the Institute of Food Science, Technology and Nutrition (ICTAN-CSIC) in Madrid. She is co-author of more than 70 papers in international scientific journals (> 6,000 citations, h-index: 34). She is also co-inventor of a patent, has been co-editor of a book published by the Royal Society of Chemistry (United Kingdom) and invited speaker in universities form United Kingdom, France, Spain, Mexico and Chile. Additionally, she was a member of the Experts Committee on Human Nutrition of the French Agency of Food Safety in 2015-18. Finally, she regularly develops dissemination activities, for which she has received several awards.
Several other researchers participated in this study: Cristina Magdaleno-Tapia, Paola Quifer-Rada, Elena Rodríguez-Rodríguez, Rocío Estévez-Santiago, Andrew L. Waterhouse, Rosa M. Lamuela-Reventós, Begoña Olmedilla-Alonso.
References
- Pérez-Jiménez, J., Arranz, S., & Saura-Calixto, F. (2009) Proanthocyanidin content in foods is largely underestimated in the literature data. An approach to quantification of missing proanthocyanidins. Food Research International, 42, 1381-88.
- Tao, W., Zhang, Y., Shen, X., Cao, Y., Shi, J., Ye, X., & Chen, S. (2019) Rethinking the Mechanism of the Health Benefits of Proanthocyanidins: Absorption, Metabolism, and Interaction with Gut Microbiota. Comprehensive Reviews Food Science & Food Safety, 18, 971-85.
- Stoupi, S., Williamson, G., Viton, F., Barron D., King L.J., Brown, J.E., & Clifford, M.N. (2010) In vivo bioavailability, absorption, excretion, and pharmacokinetics of [14C] procyanidin B2 in male rats. Drug Metabolism Disposition, 38, 287-91.
- Choy, Y.Y., Quifer-Rada, P., Holstege, D.M., Frese, S.A., Calvert, C.C., Mills, D.A., Lamuela-Raventós, R.M., & Waterhouse, A.L. (2014) Phenolic metabolites and substantial microbiome changes in pig feces by ingesting grape seed proanthocyanidins. Food and Function, 5, 2298-2308.
- Porter, L.J., Hrstich, L.N., & Chan, B.G. (1986) The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry, 25, 223-30.
- Rothwell, J.A., Fillâtre, Y., Martin, J.F., Lyan, B., Pujos-Guillot, E., Fezeu, L., Hercberg, S., Comte, B., Galán, P., Touvier, M. & Manach, C. (2014) New biomarkers of coffee consumption identified by the non-targeted metabolomic profiling of cohort study subjects. PLoS ONE, 9, Article number e93474.
- Medina-Remón, A., Barrionuevo-González, A., Zamora-Ros, R., Andrés-Lacueva, C., Estruch, R., Martínez-González, M.A., Díez-Espino, J., & Lamuela-Raventós, R.M. (2009) Rapid Folin-Ciocalteu method using microtiter 96-well plate cartridges for solid phase extraction to assess urinary total phenolic compounds, as a biomarker of total polyphenols intake. Analytica Chimica Acta, 634, 54-60.

