This post summarizes a recent review paper written by Mauro Finicelli, Anna Di Salle, Umberto Galderisi and Gianfranco Peluso and published in Nutrients. In this review the authors provided an update of the clinical trials registered on the database clinicaltrials.gov evaluating the effects of the editerranean Diet (MedDiet) on health and specific diseases.
The MedDiet is a term used to identify a dietary pattern that has caught the attention of clinicians and researchers worldwide. The MedDiet originated from the unique multi-millennial interplay between natural food resources and the eating practices of people living in the Mediterranean basin [1]. It has been widely acknowledged that the MedDiet encompasses many aspects beyond nutritional behavior, including social, cultural, economic, and environmental features. The respect for seasonality, biodiversity, and food varieties, preferring local and fresh products, is a cornerstone of the MedDiet. The association of these cultural and nutritional features with physical activity is woven into the MedDiet model, making it widely considered to be a healthy lifestyle rather than a dietary pattern [2]. UNESCO considers the MedDiet an intangible cultural heritage, given the responsible interactions between agricultural and dietary practices and the environment [3].
The term MedDiet commonly refers to the dietary pattern of the people living on the Mediterranean Sea coast, in particular Greece, southern Italy, and southern Europe [10,12]. Although these Mediterranean countries show some eating habit differences, the common features characterizing the MedDiet are defined as (a) daily consumption of non-refined cereals and other products (e.g., whole grain bread, whole grain pasta, and brown rice), fresh fruits, vegetables, nuts, and low-fat dairy products; (b) olive oil as the principal source of lipids; (c) moderate intake of alcohol, preferably red wine, with meals; (d) moderate consumption of fish, poultry, potatoes, eggs, and sweets; (e) monthly consumption of red meat; and (f) regular physical activity [8,12,13]. Besides these characteristics, the beneficial impact of the bioactive molecules in the essential individual components of the MedDiet has also been considered.
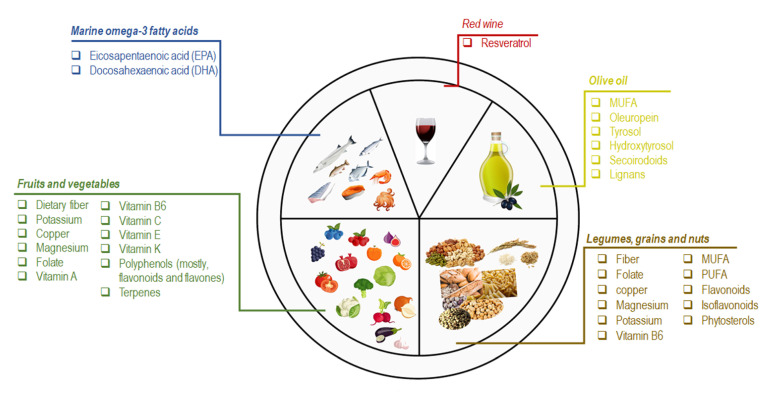
Figure: Schematic representation of the nutritional and bioactive characteristics of the principal components of the MedDiet
Marine omega-3 fatty acids are the most important bioactive molecules in fish and seafood consumed in the MedDiet (e.g., sardines, mackerel, mussels, octopus, salmon, squid, and tuna). Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are the major n-3 fatty acids recognized for their cardioprotective effects [14]. In a metaanalysis of a randomized controlled trial (RCT), EPA and DHA supplementation reduced the risk of coronary heart disease (CHD) in higher-risk populations [15]. Analogously, the high intake of fish is accountable for the beneficial effects on HDL cholesterol and triglycerides levels [16].
Olive oil (OO) and especially extra virgin olive oil (EVOO), is the primary source of fat in the MedDiet. Monosaturated fatty acids (MUFAs) are the most representative OO fatty acids (FA), ranging from 55–83% of the total FA content [17]. Saturated fatty acids (e.g., palmitate) impair mitochondrial respiration by causing an increase in both total cellular ROS and mitochondrial ROS, contrary to MUFAs, such as oleate from OO [18,19].

Nevertheless, controversies still exist on the beneficial effect of MUFA on human health. On the other hand, much attention must be given to the lesser components of OO (2% of the total weight), consisting of polyphenols or other secondary plant metabolites (e.g., oleuropein, tyrosol, hydroxytyrosol, secoirodoids, and lignans) [20,21]. Overall, these molecules contribute to the beneficial effects of OO and EVOO on human health. A meta-analysis of 32 observational studies revealed that OO consumption decreased the risk of stroke, CHD, and diabetes and improved some metabolic and inflammatory biomarkers [22–24]. The consumption of OO enriched in phenolic compounds seemed to reduce the urinary levels of DNA oxidation (8-OHdG) and inflammatory (IL-8, TNF-α) markers in an opportune population cohort [25]. The regular intake of 15 mg/day of HTyr resulted in modification of body composition parameters and modulated the antioxidant profile, the expression of inflammation and oxidative stress-related genes in atherosclerosis [26].
Fruit and vegetable consumption is emphasized in the MedDiet. Oranges, pomegranates, berries, figs, and grapes are the most frequently consumed fruits and a source of dietary fiber, potassium, vitamin C, polyphenols (mostly flavones), and terpenes [8]. Vegetables are mainly seasonal and field-grown. The most representative vegetables are fresh greens, tomatoes, eggplants, cabbages, radishes, garlic, onions, spinach, and lettuce. Although these foods are an essential source of nutrients (e.g., dietary fiber, potassium, copper, magnesium, folate, vitamin-A, -B6, -C, -E, -K), the phenolic compounds (mainly flavonoids) are the most important bioactive molecules [27,28]. Several studies demonstrated that high consumption of vegetables or fruit resulted in lower risk for all-cause mortality, CHD, stroke, T2D, colon rectal cancer (CRC), and adiposity [16,29,30,31,32,33].
Legumes, grains, and nuts are regularly consumed in the MedDiet. Grains appear both as a single food (e.g., rice and oatmeal) and as ingredients of processed foods (e.g., bread, pasta, cereal, and crackers). Common MedDiet nuts include almonds, hazelnuts, walnuts, and pistachios. Among legumes, the most representative is lentils, beans, and chickpeas. Overall, these foods are a valuable source of fiber, folate, vitamin B6, magnesium, potassium, and copper [27,30]. In particular, nut intake is crucial because these foods are unique for their MUFAs and polyunsaturated FA (PUFAs) content, especially linoleic, linolenic acid, phenols, flavonoids, isoflavonoids, and phytosterols [12,34]. The beneficial effect of nut consumption primarily impacts the incidence of CVD, diabetes, and MetS [35,36,37]. The beneficial effects of legumes and grains on CVD, body weight, and cholesterol (total and LDL-C) have also been described. Of note, grain consumption has also been associated with a lower risk of T2B and CRC [16,29,30,31,32,33].
Red wine is routinely consumed MedDiet meals. Resveratrol is the most abundant polyphenol in red wine. Besides the acknowledged effect of this molecule on several chronic diseases (e.g., cancer, myocardial infarction, and brain disorders), evidence supports its role in protecting against the development of some MetS features [12,38].
Although this body of evidence demonstrates the role of nutrients and foods in the pathophysiology of numerous diseases, the mechanisms by which the MedDiet exerts its favorable effects are not fully understood. However, the five most important influences induced by adherence to the MedDiet can be summarized as (a) lipid-lowering effect, (b) anti-oxidative and anti-inflammatory action, (c) modification of key molecules (hormones and growth factors) involved in the pathogenesis of cancer, (d) inhibition of nutrient-sensing pathways, and (e) gut microbiota-mediated production of metabolites influencing metabolic health [39]. The current research focuses on the MedDiet pattern as a whole. The overall combination of MedDiet foods and their components’ additive or synergistic effects seems to provide more consensus regarding health benefits [3]. Moreover, food production, consumption, cooking techniques, and eating behaviors are also emerging as innovative variables used to assess the beneficial effects of the MedDiet [40]. Accordingly, research must go beyond the common links between foods and nutrients and consider other aspects characterizing the MedDiet as a healthy lifestyle.
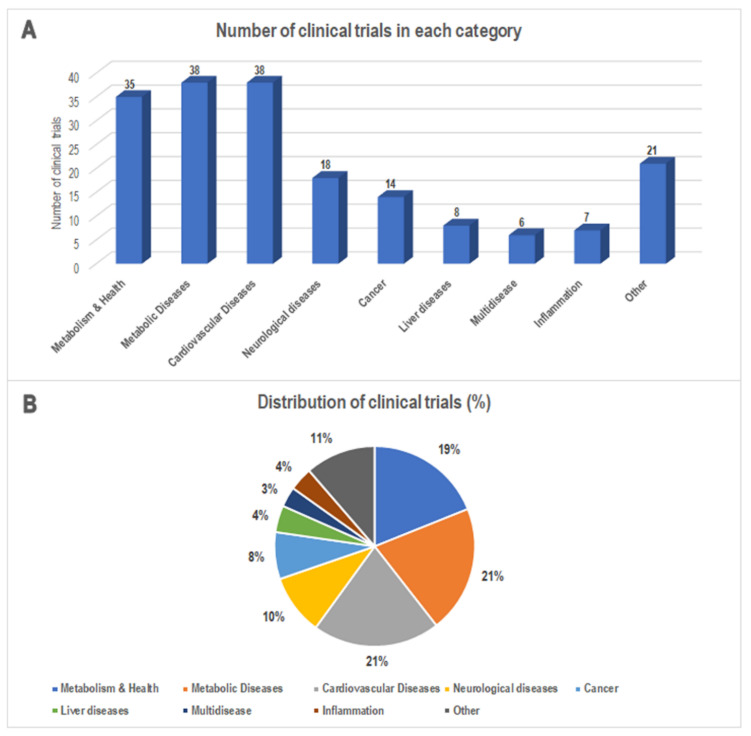
Figure: (A) The number and (B) percentage of MedDiet-based clinical trials classified according to the indications provided by the clinical trials database. Data from http://www.clinicaltrials.gov/ (accessed on 31 January 2022).
The authors provided an update of the clinical trials in which the direct or indirect effects of the MedDiet were evaluated on health and specific diseases. The importance of the results obtained by MedDiet-based trials is well acknowledged both for clinical and social implications. Their findings revealed an increase in the number of clinical trials in the last decade that pairs with the enhanced level and quality of the evidence concerning the effects of this dietary pattern [2]. They found that the most representative diseases challenged with the MedDiet are cardiovascular diseases, metabolic diseases, and cancer. This is proof of the beneficial potential of this dietary pattern on chronic diseases affecting people worldwide. Nevertheless, a discrepancy exists between the increased number of completed clinical trials and the few published results (positive or negative). This should be better addressed since these data could be instrumental in preventing potentially inefficacious pleonastic clinical trials and providing novel evidence-driven studies.
In summary, the studies’ findings disclose that the MedDiet’s beneficial effects could be primarily related to its anti-inflammatory and antioxidant properties. The effectiveness of this dietary pattern in controlling waist circumference and obesity seems to be another key aspect. Moreover, strict, and long-lasting adherence to the MedDiet as well as the beneficial effects of specific components (e.g., olive oil or its polyphenols) seem to emerge as useful insights for interventional improvements. The synergic effect of the MedDiet and physical exercise has also been exanimated, mainly in cancer survivor patients. This is proof of the emerging evidence that addresses the MedDiet as a healthy lifestyle rather than a simple dietary pattern. Moreover, the intriguing interaction between MedDiet’s components and the immune system will pave the way for tailored studies aiming to dissect this complex interplay. These findings will provide further insights useful for the clinical practice.
The MedDiet has recently emerged as a suitable model of sustainable nutrition, in which nutrition, local foods, biodiversity, culture, and sustainability are strictly interconnected. Dietary habits are drivers of environmental pressure, and it seems that the MedDiet could beneficially impact this aspect. The low environmental impact of the MedDiet with respect to other dietary patterns has been estimated regarding water consumption, nitrogen emission, and carbon footprint [2,3]. Indeed, the MedDiet is essentially a plant-based diet with few animal products, mainly red meat consumed monthly. Animal-based foods are the most land-and-energy intensive. On the other hand, vegetables, cereals, nuts, and olive oil lower GHG emissions.
In conclusion, this evidence demonstrates the importance of the MedDiet as the combination of a healthy dietary pattern and healthy behaviors. The conveyance of this model toward other populations and countries is desirable to export its beneficial implications both for clinical and environmental aspects. Moreover, evidence from long-term studies is needed to sustain the promising data described here and further dissect the functional implications of the MedDiet for chronic pathologies such as cardiovascular diseases, metabolic diseases, and cancer. This is a suitable approach to defining the MedDiet’s beneficial contribution to risk factors and other outcomes related to these diseases.
Read all paper at: https://www.mdpi.com/2072-6643/14/14/2956
References
- Lacatusu C.M., Grigorescu E.D., Floria M., Onofriescu A., Mihai B.M. The Mediterranean Diet: From an Environment-Driven Food Culture to an Emerging Medical Prescription. Int. J. Environ. Res. Public Health. 2019;16:942. doi: 10.3390/ijerph16060942.
- Serra-Majem L., Ortiz-Andrellucchi A., Sánchez-Villegas A. Mediterranean Diet. In: Ferranti P., Berry E.M., Anderson J.R., editors. Encyclopedia of Food Security and Sustainability. Elsevier; Oxford, UK: 2019. pp. 292–301.
- Guasch-Ferre M., Willett W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021;290:549–566. doi: 10.1111/joim.13333.
- Wright C.M. Biographical notes on Ancel Keys and Salim Yusuf: Origins and significance of the seven countries study and the INTERHEART study. J. Clin. Lipidol. 2011;5:434–440. doi: 10.1016/j.jacl.2011.09.003.
- Keys A., Taylor H.L., Blackburn H., Brozek J., Anderson J.T., Simonson E. Coronary Heart Disease among Minnesota Business and Professional Men Followed Fifteen Years. Circulation. 1963;28:381–395. doi: 10.1161/01.CIR.28.3.381.
- Keys A., Menotti A., Karvonen M.J., Aravanis C., Blackburn H., Buzina R., Djordjevic B.S., Dontas A.S., Fidanza F., Keys M.H., et al. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 1986;124:903–915. doi: 10.1093/oxfordjournals.aje.a114480.
- de Lorgeril M. Mediterranean diet and cardiovascular disease: Historical perspective and latest evidence. Curr. Atheroscler. Rep. 2013;15:370. doi: 10.1007/s11883-013-0370-4.
- Schwingshackl L., Morze J., Hoffmann G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2020;177:1241–1257. doi: 10.1111/bph.14778.
- Dinu M., Pagliai G., Casini A., Sofi F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018;72:30–43. doi: 10.1038/ejcn.2017.58.
- Corella D., Coltell O., Macian F., Ordovas J.M. Advances in Understanding the Molecular Basis of the Mediterranean Diet Effect. Annu. Rev. Food Sci. Technol. 2018;9:227–249. doi: 10.1146/annurev-food-032217-020802.
- Kargin D., Tomaino L., Serra-Majem L. Experimental Outcomes of the Mediterranean Diet: Lessons Learned from the Predimed Randomized Controlled Trial. Nutrients. 2019;11:2991. doi: 10.3390/nu11122991.
- Finicelli M., Squillaro T., Di Cristo F., Di Salle A., Melone M.A.B., Galderisi U., Peluso G. Metabolic syndrome, Mediterranean diet, and polyphenols: Evidence and perspectives. J. Cell. Physiol. 2019;234:5807–5826. doi: 10.1002/jcp.27506.
- Gouveri E., Diamantopoulos E.J. The Mediterranean diet andmetabolic syndrome. In: Preedy V., Watson R., editors. The Mediterranean Diet. Elsevier Inc.; Cambridge, UK: 2015. pp. 313–323.
- Wang C., Enssle J., Pietzner A., Schmocker C., Weiland L., Ritter O., Jaensch M., Elbelt U., Pagonas N., Weylandt K.H. Essential Polyunsaturated Fatty Acids in Blood from Patients with and without Catheter-Proven Coronary Artery Disease. Int. J. Mol. Sci. 2022;23:766. doi: 10.3390/ijms23020766.
- Alexander D.D., Miller P.E., Van Elswyk M.E., Kuratko C.N., Bylsma L.C. A Meta-Analysis of Randomized Controlled Trials and Prospective Cohort Studies of Eicosapentaenoic and Docosahexaenoic Long-Chain Omega-3 Fatty Acids and Coronary Heart Disease Risk. Mayo Clin. Proc. 2017;92:15–29. doi: 10.1016/j.mayocp.2016.10.018.
- Schwingshackl L., Schlesinger S., Devleesschauwer B., Hoffmann G., Bechthold A., Schwedhelm C., Iqbal K., Knuppel S., Boeing H. Generating the evidence for risk reduction: A contribution to the future of food-based dietary guidelines. Proc. Nutr. Soc. 2018;77:432–444. doi: 10.1017/S0029665118000125
- Finicelli M., Squillaro T., Galderisi U., Peluso G. Polyphenols, the Healthy Brand of Olive Oil: Insights and Perspectives. Nutrients. 2021;13:3831. doi: 10.3390/nu13113831.
- Di Cristo F., Finicelli M., Digilio F.A., Paladino S., Valentino A., Scialo F., D’Apolito M., Saturnino C., Galderisi U., Giordano A., et al. Meldonium improves Huntington’s disease mitochondrial dysfunction by restoring peroxisome proliferator-activated receptor gamma coactivator 1alpha expression. J. Cell. Physiol. 2019;234:9233–9246. doi: 10.1002/jcp.27602.
- Joseph L.C., Barca E., Subramanyam P., Komrowski M., Pajvani U., Colecraft H.M., Hirano M., Morrow J.P. Inhibition of NAPDH Oxidase 2 (NOX2) Prevents Oxidative Stress and Mitochondrial Abnormalities Caused by Saturated Fat in Cardiomyocytes. PLoS ONE. 2016;11:e0145750. doi: 10.1371/journal.pone.0145750.
- Rodriguez-Lopez P., Lozano-Sanchez J., Borras-Linares I., Emanuelli T., Menendez J.A., Segura-Carretero A. Structure-Biological Activity Relationships of Extra-Virgin Olive Oil Phenolic Compounds: Health Properties and Bioavailability. Antioxidants. 2020;9:685. doi: 10.3390/antiox9080685.
- Karkovic Markovic A., Toric J., Barbaric M., Jakobusic Brala C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules. 2019;24:2001. doi: 10.3390/molecules24102001
- Schwingshackl L., Schwedhelm C., Galbete C., Hoffmann G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients. 2017;9:1063. doi: 10.3390/nu9101063.
- Schwingshackl L., Christoph M., Hoffmann G. Effects of Olive Oil on Markers of Inflammation and Endothelial Function—A Systematic Review and Meta-Analysis. Nutrients. 2015;7:7651–7675. doi: 10.3390/nu7095356.
- Schwingshackl L., Hoffmann G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014;13:154. doi: 10.1186/1476-511X-13-154.
- Sanchez-Rodriguez E., Biel-Glesson S., Fernandez-Navarro J.R., Calleja M.A., Espejo-Calvo J.A., Gil-Extremera B., de la Torre R., Fito M., Covas M.I., Vilchez P., et al. Effects of Virgin Olive Oils Differing in Their Bioactive Compound Contents on Biomarkers of Oxidative Stress and Inflammation in Healthy Adults: A Randomized Double-Blind Controlled Trial. Nutrients. 2019;11:626. doi: 10.3390/nu11030561.
- Colica C., Di Renzo L., Trombetta D., Smeriglio A., Bernardini S., Cioccoloni G., Costa de Miranda R., Gualtieri P., Sinibaldi Salimei P., De Lorenzo A. Antioxidant Effects of a Hydroxytyrosol-Based Pharmaceutical Formulation on Body Composition, Metabolic State, and Gene Expression: A Randomized Double-Blinded, Placebo-Controlled Crossover Trial. Oxid. Med. Cell. Longev. 2017;2017:2473495. doi: 10.1155/2017/2473495.
- Delgado A.M., Vaz Almeida M.D., Parisi S. Chemistry of the Mediterranean Diet. Springer; Cham, Switzerland: 2017.
- Hoffman R., Gerber M. Evaluating and adapting the Mediterranean diet for non-Mediterranean populations: A critical appraisal. Nutr. Rev. 2013;71:573–584. doi: 10.1111/nure.12040.
- Bechthold A., Boeing H., Schwedhelm C., Hoffmann G., Knuppel S., Iqbal K., De Henauw S., Michels N., Devleesschauwer B., Schlesinger S., et al. Food groups and risk of coronary heart disease, stroke and heart failure: A systematic review and dose-response meta-analysis of prospective studies. Crit. Rev. Food. Sci. Nutr. 2019;59:1071–1090. doi: 10.1080/10408398.2017.1392288.
- Schlesinger S., Neuenschwander M., Schwedhelm C., Hoffmann G., Bechthold A., Boeing H., Schwingshackl L. Food Groups and Risk of Overweight, Obesity, and Weight Gain: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2019;10:205–218. doi: 10.1093/advances/nmy092.
- Schwingshackl L., Hoffmann G., Lampousi A.M., Knuppel S., Iqbal K., Schwedhelm C., Bechthold A., Schlesinger S., Boeing H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017;32:363–375. doi: 10.1007/s10654-017-0246-y. [
- Schwingshackl L., Schwedhelm C., Hoffmann G., Lampousi A.M., Knuppel S., Iqbal K., Bechthold A., Schlesinger S., Boeing H. Food groups and risk of all-cause mortality: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2017;105:1462–1473. doi: 10.3945/ajcn.117.153148.
- Schwingshackl L., Schwedhelm C., Hoffmann G., Knuppel S., Iqbal K., Andriolo V., Bechthold A., Schlesinger S., Boeing H. Food Groups and Risk of Hypertension: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2017;8:793–803. doi: 10.3945/an.117.017178.
- Manach C., Scalbert A., Morand C., Remesy C., Jimenez L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727.
- Zec M., Glibetic M. Reference Module in Food Science. Elsevier; Oxford, UK: 2018. Health Benefits of Nut Consumption.
- Kelly J.H., Jr., Sabate J. Nuts and coronary heart disease: An epidemiological perspective. Br. J. Nutr. 2006;96((Suppl. 2)):61–67. doi: 10.1017/BJN20061865.
- Jiang R., Jacobs D.R., Jr., Mayer-Davis E., Szklo M., Herrington D., Jenny N.S., Kronmal R., Barr R.G. Nut and seed consumption and inflammatory markers in the multi-ethnic study of atherosclerosis. Am. J. Epidemiol. 2006;163:222–231. doi: 10.1093/aje/kwj033.
- Opie L.H., Lecour S. The red wine hypothesis: From concepts to protective signalling molecules. Eur. Heart J. 2007;28:1683–1693. doi: 10.1093/eurheartj/ehm149.
- Tosti V., Bertozzi B., Fontana L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018;73:318–326. doi: 10.1093/gerona/glx227. [PMC free article] [
- Serra-Majem L., Roman-Vinas B., Sanchez-Villegas A., Guasch-Ferre M., Corella D., La Vecchia C. Benefits of the Mediterranean diet: Epidemiological and molecular aspects. Mol. Asp. Med. 2019;67:1–55. doi: 10.1016/j.mam.2019.06.001.

