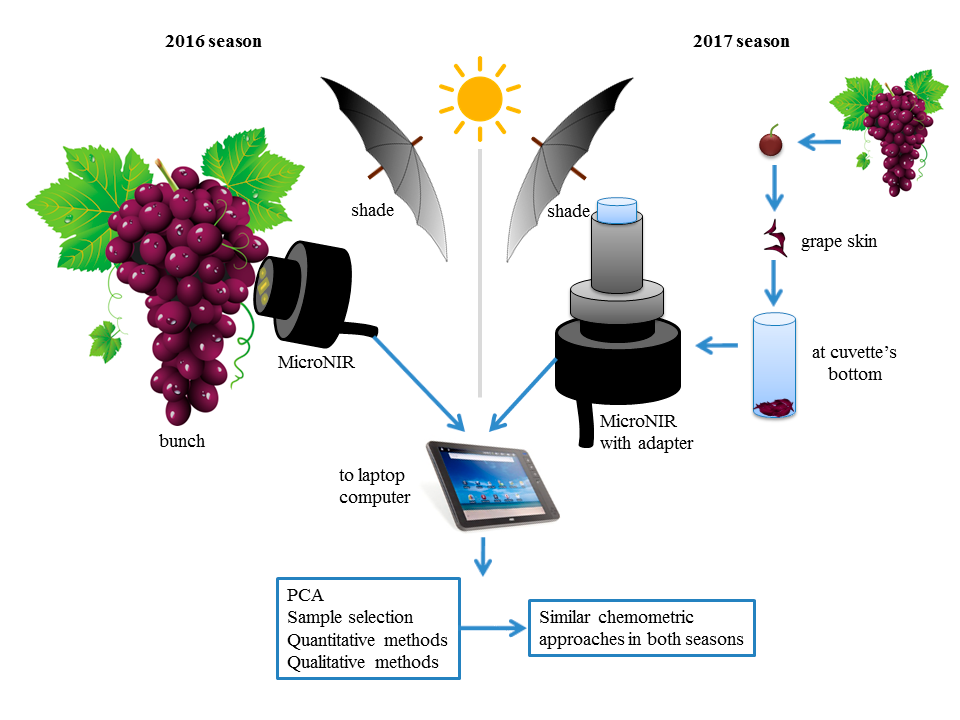
Sparkling wine production comprises two successive fermentations performed by Sacharomyces cerevisiae strains. This post is about a project aimed to develop yeast immobilisation processes on two wine-compatible supports, study the effects of yeast type (IOC 18–2007 and 55A) and the immobilisation support type (oak chips and cellulose powder) on the fermentation kinetics, the deposition rate of lees and the volatile composition of the finished sparkling wine; compare the fermentation parameters of the wines inoculated with immobilised or non-immobilised cells.
According with the last post of the year the use of immobilization supports reports the advantages of rapid yeast elimination and not adding bentonite and does not have a negative impact on the wine sensory profile.