By Carmen Berbegal and Isabel Pardo
High quality sparkling wines using the traditional or “champenoise” method include two fermentations carried out by Saccharomyces cerevisiae (Garofalo et al. 2018). The starter cultures that are used to develop the second fermentation inside the capped bottles need to possess several additional technological properties to those yeasts used in the first fermentation. They have to be selected in order to survive in stress conditions, in particular this yeast must be chosen for its ability to ferment high-acidity and low-pH wines, and it must be ethanol-tolerant (Garofalo et al. 2016). A way to protect yeast cells from stress in the second fermentation is through the cell immobilization (Nedović et al. 2015). Moreover, this mechanism can reduce the use of riddling agents. Thus, the objectives of our project were to initially develop yeast immobilization processes on two wine-compatible supports: oak chips and cellulose powder; secondly, to study the effects of type of yeast and type of immobilization support on the fermentation kinetics, the deposition rate of lees and the volatile composition of finished sparkling wine; finally, to compare the fermentation parameters of the wines inoculated with immobilized or non-immobilized cells.
We immobilized two different Saccharomyces cerevisiae yeast strains in oak chips and cellulose powder by lyophilization: the Saccharomyces cerevisiae strain Enolab55A (55A) isolated from an organic Spanish wine from Utiel-Requena D.O.P. in Spain and the commercial yeast IOC18-2007. As shown in Figure 1, the lyophilized Saccharomyces cerevisiae cells were adhered to the surfaces of oak chips/cellulose powder.
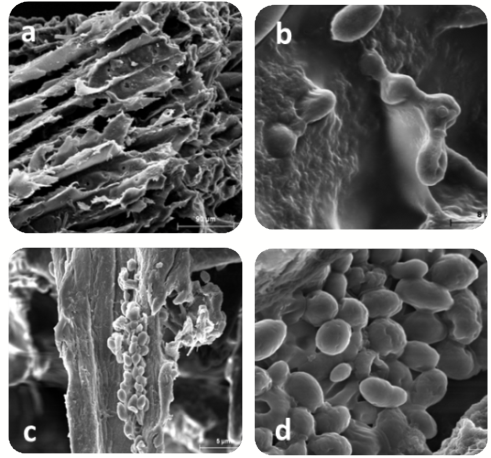
All the vinification trials were run in a base wine that consisted in a coupage of 80% Macabeo and 20% Chardonnay. We distributed the base wine in transparent glass bottles, the “tirage liqueur” and we added the immobilized oak chips/cellulose powder yeasts (1 g/L), or a free cell yeast culture (2×106 CFU/mL) (Fig. 2). No riddling agents were added. Each week during the first month and at the end of the second month of ageing, we opened three bottles per experiment to determine the residual sugars and ethanol concentrations (Frayne et al. 1986). After 9 months, we measured yeast sediment deposition efficiency by considering the total time that the gyropalette required to make wines transparent and we determined the volatile composition of the resulting sparkling wines at the end of the ageing period (Ortega et al. 2011).
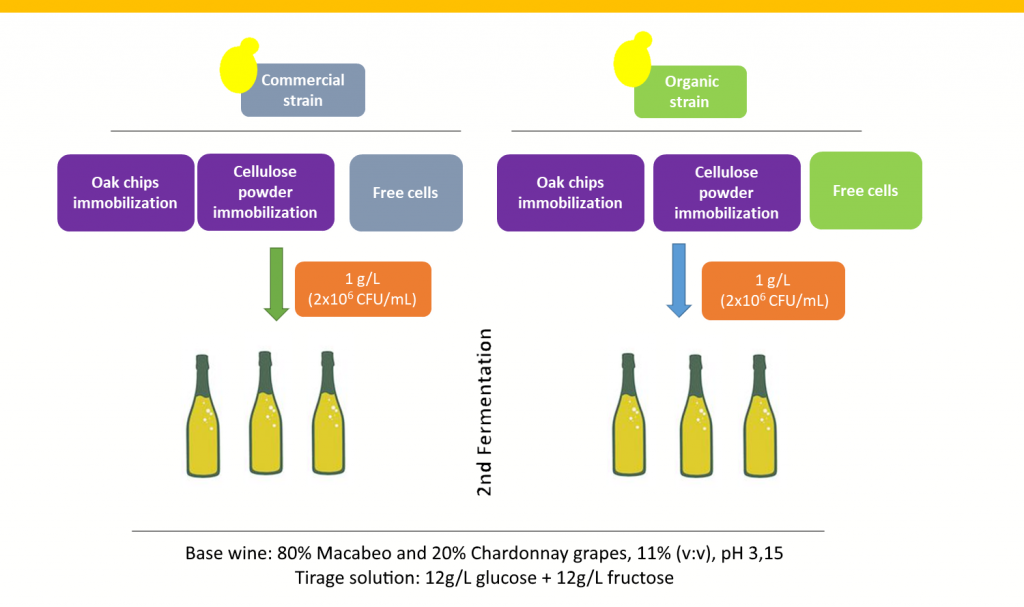
Our results showed that the final sugar and ethanol concentrations were similar in the sparkling wines fermented with the free cells or those immobilized on oak chips/cellulose powder cells, regardless of the yeast strain used; glucose and fructose were consumed in under 60 days and ethanol increased by 1.5% with the second fermentation. During the riddling performed automatically with a gyropalette, the immobilized yeasts on oak chips/cellulose powder settled in the neck of the bottles 3-fold faster than in the bottles that contained free cells. Completely transparent wines were obtained without having to resort to riddling agents, such as bentonite, which results in less manipulated and more natural products (Figure 3).

When the volatile aroma analysis was done, thirty-two volatile compounds were identified in sparkling wines. We found significant differences in the formation of esters, acids, alcohols, aldehydes and lactones depending on the yeast used and the immobilization support. From both supports, oak chips were the more appropriate support for yeast immobilization. Despite the differences observed in the profile of volatile wines, at the sensory level no significant differences appeared between the fermented wines with both yeast types and those with the free or immobilized yeast with the different immobilization substrates.
It is important to highlight that during the sparkling wine-making process, the second fermentation occurs under very particular conditions for yeasts; the base wine has high alcohol content, and not all strains can grow and ferment under these conditions. Our results suggest that this new technology can be used with the organic yeast 55A as an alternative to commercial starter IOC18-2007, and that the use of immobilization supports reports the advantages of rapid yeast elimination and not adding bentonite, and does not have a negative impact on the wine sensory profile.
Those interested in a longer length report can download the working paper at:
https://www.sciencedirect.com/science/article/pii/S0740002017311942
This work was supported by the “Programa Valoritxa i Transfereix” 2013 (Ref: UV-CPI13274-159983) of the Universitat de València and carried out by Carmen Berbegal, Lucía Polo, Mª José García-Esparza, Victoria Lizama, Sergi Ferrer, Isabel Pardo and people from the winery ‘Dominio de la Vega’.
Carmen Berbegal is a PostDoc researcher from Enolab group at the University of Valencia (Spain). In addition to the University of Valencia education, she has been formed as a microbiologist in the National Collection of Industrial and Marine Bacteria (Scotland), in the University of Patras (Greece) and in the University of Foggia (Italy). All her investigations are focused on wine microbiology, mainly on lactic acid bacteria and on applied aspects related to the use of microorganisms to solve technological problems in the wine industry. She has been involved in the characterization and selection of lactic bacteria such as Oenococcus oeni and Lactobacillus plantarum to design different starter cultures to improve ‘wine qualities’, to avoid the growth of undesirable microorganisms, and to study the formation and the control of undesirable toxic compounds produced by lactic acid bacteria, such as biogenic amines. Reserchgate profile: https://www.researchgate.net/profile/Carmen_Berbegal , Scholar profile: https://scholar.google.it/citations?user=pLRzzcMAAAAJ&hl=it

Isabel Pardo holds a degree in Biology and a PhD in Biology from the University of Valencia. She obtained the position of Full Professor at the Univ. Valencia in 2012. Co-author of more than 140 scientific papers and 240 congress communications. Regular editor/reviewer of many scientific and technical journals related to Microbiology and Oenology. Supervisor of 12 doctoral theses, one of them with Extraordinary Doctorate Prize, and supervisor of graduate/postgraduate students. Participation in 57 R+D+i Projects financed in competitive calls of Regional Administrations or public and private entities (in 9 of them as Principal Researcher), and in not competitive 37 Contracts, research agreements or R&D+i projects of Regional Administrations or public and private entities (in 7 of them as Principal Researcher). Co-author of a Spanish patent and of another International. She is member of different networks and associations related to microbiology, food and wine: CYTED, Gienol, Microcluster Vitivinicultura, Technological Platform of Wine, RedBal, Red Sicura. She is an evaluator of the National Agency for Evaluation and Prospective (ANEP) that depends on the Secretariat of State for Research, Ministry of Science and Innovation of Spain. She has extensive in Wine Microbiology.
References:
- Frayne, R.F., 1986. Direct analysis of the major organic components in grape must and wine using high performance liquid chromatography. Am. J. Enol. Vitic. 37, 281-287.
- Garofalo, C., Arena, M., Laddomada, B., Cappello, M., Bleve, G., Grieco, F., Beneduce, L., Berbegal, C., Spano, G., Capozzi, V., 2016. Starter cultures for sparkling wine. Fermentation 2, 21.
- Garofalo, C., Berbegal, C., Grieco, F., Tufariello, M., Spano, G., Capozzi, V., 2018. Selection of indigenous yeast strains for the production of sparkling wines from native Apulian grape varieties. Int J Food Microbiol 285, 7-17.
- Nedović, V., Gibson, B., Mantzouridou, T.F., Bugarski, B., Djordjević, V., Kalušević, A., Paraskevopoulou, A., Sandell, M., Šmogrovičová, D., Yilmaztekin, M., 2015. Aroma formation by immobilized yeast cells in fermentation processes. Yeast 32, 173-216.
- Ortega, C., Lopez, R., Cacho, J., Ferreira, V., 2001. Fast analysis of important wine volatile
- compounds development and validation of a new method based on gas chromatographic- flame ionisation detection analysis of dichloromethane microextracts. J. Chromatogr. A 923, 205–214.

