By Suzy Rogiers and Francesca Moroni
What is chronobiology?
Chronobiology refers to the periodic rhythms of organisms in response to solar or lunar cycles. Chronobiology is a phenomenon that occurs in many behavioural, physiological and metabolic processes of living organisms. Cycling rhythms in biological organisms can occur with change in seasons (such as flower development or hibernation), to lunar-monthly (marine animal breeding), to changes that cycle approximately every 24 hours (the sleep-wake cycle in many animals), to cycles that last less than 24 hours (REM periods during sleep). Some of these cycles are driven by changes in the external environment while others are endogenous and not dependent on external stimuli.

Biological clocks in the vineyard
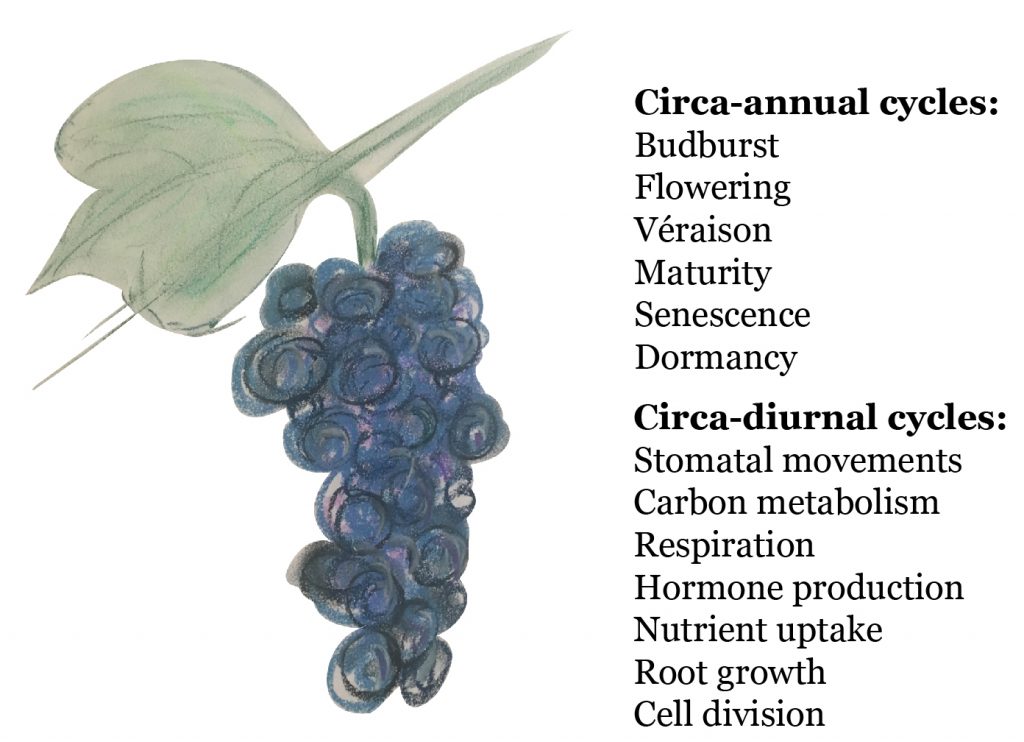
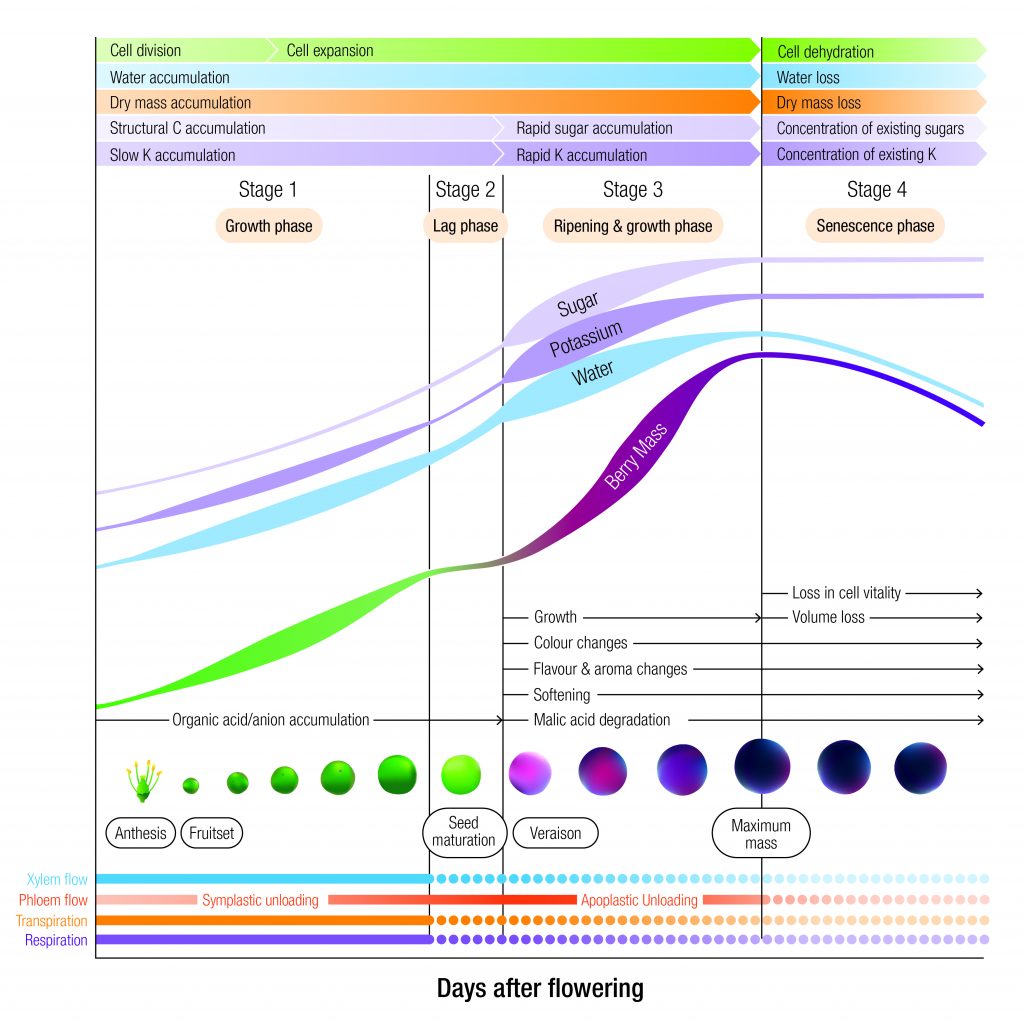
Evidence for chronobiology can be found in many aspects of viticulture. Buds swell and burst every spring with warmer temperatures and longer days. The onset of flowering is also driven by changes in day length and its timing is important to reproductive success. Grapevines can sense longer days in spring through the light receptors located in green tissues. Alternatively, leaf senescence is activated as day length shortens in autumn and the vine prepares for dormancy. Co-ordination between cells and tissues of the shoot tips, leaves, woody components and roots is critical to successful regulation of growth and development (Figure 1). This may explain the presence of multiple clocks in different tissue types within any one organism.
The vineyard is a complex ecosystem with its resident and visiting organisms inherently undertaking seasonal and daily adjustments to the changing surroundings. For instance seasonal bird migration into and out of the vineyard is one aspect of an annual cycle. These same birds will likely have diurnal rhythms in eating behaviour and digestion, body temperature, immunity response and hormone release among others. Nocturnal grazing of insects and caterpillars on succulent leaf tissues revolve around a 24 h cycle. Butterflies are active during the day while moths are active at night. Mushrooms and other fungi release spores either diurnally or nocturnally. Flowers of weeds growing on the vineyard floor may only release scent molecules during a specific time of to conserve energy. Honeybees visiting these flowers are trained to feed on them at that specific time of day. The same weeds undergo photosynthesis and stomatal movements in a diurnal cycle. Even the microbes living on the berry surface likely undergo rhythmic changes in metabolic processes.
Circadian rhythms
The approximate 24 hour endogenous cycle is referred to as a circadian rhythm and is common in plants. The daily tracking of the sun by leaves of the mimosa plant (first described in 1729 by French astronomer Jean Jacques d’Ortous de Mairan), the opening and closing of tulip petals, the tracking of the sun by the sunflower, and carbohydrate metabolism are good examples of this type of rhythm. Circadian rhythms are driven by a self-sustained 24 h internal clock. These rhythms persist for some time despite the absence of environmental cues such as changes in light or temperature, but they are able to adjust (entrain) to these stimuli. For instance, the opening and closing of a flower is circadian, not just diurnal, if it continues after the plant is placed in continuous darkness. However, after some time the rhythm is lost, illustrating the importance of environmental cues in maintaining the rhythm. Circadian rhythms gives an organism the competitive advantage of ‘anticipating‘ change in its environment so that it can respond appropriately. For examples, Charles Darwin and his son Francis (1880) hypothesised that circadian leaf movements might be important for heat transfer. They found that a vertical leaf position reduces heat loss at night so that the plants are less likely to freeze.
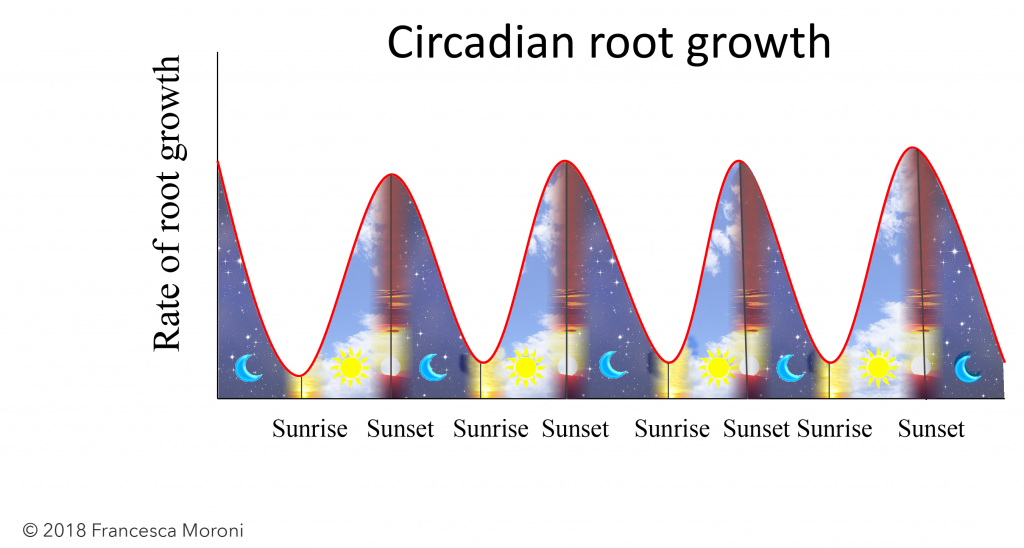
Circadian root growth
Growth of plant organs can also follow circadian rhythms. The elongation of cells in leaves and stems can follow such oscillations. This and much other circadian behaviour are under transcriptional and translational regulation with underlying multiple interlocked feedback loops.
In plant science, root growth studies are not as common as those of the above ground components due to the difficulty of monitoring roots in the soil medium; this is especially challenging for large perennial plants with widespread root systems that grow far and deep. These difficulties have been partially overcome by rhizotrons (transparent windows placed in the soil) with scanners and cameras that allow frequent image acquisition. Using such methods we have shown that root growth in grapevines follows a diurnal rhythm with a maximum at sunset and a minimum at sunrise, despite constant soil temperature and moisture (Figure 2). Slow root growth by the end of the night can be explained by the depletion of starch reserves from the roots. Stressful soil temperatures that are too warm will dampen the maximum rate of root growth but will not shift its diurnal pattern.
Exposing vines to shorter daylight in controlled environment chambers also did not disrupt the diurnal pattern demonstrating that root growth is under circadian control. Interestingly, diurnal shoot growth patterns did shift with changes in day length indicating that, in grapevines, shoot elongation is not under endogenous regulation.
There is no doubt that temperature and light are critical drivers for grape berry growth and development and leaf photosynthesis provides the carbon source to drive these processes. However the hidden half of the vine is integral to water and nutrient uptake and a better understanding of the environmental and endogenous factors that impinge on root functioning will result in better wines.
Biological clocks and wine perception
We have demonstrated that grapevine biological clocks will impinge on berry development and thus berry composition and the resultant wine style, but do human biological clocks have an impact on how a wine is perceived? The subjective experience of a wine is influenced by appetite, time of day and the physical and social environment of the taster. How our innate circadian rhythms interact with our ability to perceive, decode and process the sensory stimuli offered by wine may well influence our physiological and behavioural response. Little is known about how circadian clocks control taste and other chemosensory systems but hopefully some light will be shed on this multifaceted subject so that we may enjoy our wine to the fullest.
Those interested in a longer length report can download the working paper at:
https://www.sciencedirect.com/science/article/pii/S017616171830018X
Suzy Rogiers is Principal Research Scientist with NSW Department of Primary Industries. She is also adjunct Professor at Western Sydney University and a member of the National Wine & Grape Industry Centre in Australia. Her research is focussed on the impacts of heat, drought and unpredictable weather on crop productivity. She has researched water-use efficiency, nutrition and carbon acquisition in horticultural crops with a focus on wine grapes. She has a particular interest in fruit colour, flavour, aroma and nutritional profile in response to environmental conditions (light, temperature, water) and cultural practises (canopy management, orchard floor management, irrigation, nutrition, clonal selection and rootstocks). She is also interested in the endogenous regulation of fruit development and chronobiology.
Francecsa Moroni is a Sydney based artist and student of Design in Architecture. She composed the images for this article.
References:
- Bordage, S., Sullivan, S., Laird, J., Millar, A.J., Nimmo, H.G., 2016. Organ specificity in the plant circadian system is explained by different light inputs to the shoot and root clocks. New Phytol. 212, 136–149.Clarke, S.J., Lamont, K.J., Pan, H.Y., Barry, L.A., Hall, A., Rogiers, S.Y., 2015. Spring rootzone temperature regulates root growth, nutrient uptake and shoot growth dynamics in grapevines. Austr. J. Grape Wine Res. 21, 479–489.
- James, A.B., Monreal, J.A., Nimmo, G.A., Kelly, C.L., Herzyk, P., Jenkins, G.I., Nimmo, H.G., 2008. The circadian clock in Arabidopsis roots is a simplified slave version of the clock in shoots. Science 322, 1832–1835.
- Mahmud ,K.P., Holzapfel, B.P., Smith, J.P., Guisard, Y., Nielsen S., Rogiers, S.Y. 2018. Circadian regulation of grapevine root and shoot growth and their modulation by photoperiod and temperature. J.Plant Physiol. 222: 86-93.
- Mahmud, K.P., Smith, J.P., Rogiers, S.Y., Guisard, Y., Nielsen, S., Holzapfel, B.P. 2018. Diurnal Root Growth Dynamics in Mature Grapevines. Acta Hort. 1205: 555-562.
- Mora-García, S., de Leone, M.J., Yanovsky, M., 2017. Time to grow: circadian regulation of growth and metabolism in photosynthetic organisms. Curr. Opin. Plant Biol. 35,84–90.
- Nozue, K. and Maloof, J.N., 2006. Diurnal regulation of plant growth. Plant, Cell Environ. 29, 396-408.
- Simon, N.M.L., Dodd, A.N., 2017. A new link between plant metabolism and circadian rhythms? Plant Cell Environ. 40, 995–996.
- Yazdanbakhsh, N., Sulpice, R., Graf, A., Stitt, M., Fisahn, J., 2011. Circadian control of root elongation and C partitioning in Arabidopsis thaliana. Plant Cell Environ. 34,877–894.