By Francesco Fancello, Chiara Multineddu, Mario Santona, Maria Giovanna Molinu, Giacomo Zara, Sandro Dettori, Pierfrancesco Deiana, Severino Zara
Among the edible vegetable oils, virgin olive oil (VOO) is one of the few to be consumed unprocessed, and it is widely considered one of the world’s healthiest foods. Indeed, oil extraction is exclusively carried out through mechanical and physical processes. This permits the retention of all the fruit’s nutrients and antioxidants, as well as the preservation of its aroma and sensory properties. Its lack of processing also means that VOO provides an important source of bioactive components, such as phenolic compounds, sterols, vitamins, and squalene, with their associated antioxidant, anti-inflammatory, and antimicrobial activities (Brenes, Medina, Romero, & de Castro, 2007; D’Angelo et al., 2005; Medina et al., 2006; Visioli et al., 2005; Yakhlef et al., 2018). Due to the high concentration of monounsaturated fatty acids (oleic acid), and the presence of phytochemical components, VOO exerts beneficial effects on human health and has thus been considered a real functional food.
The phenolic compounds present in olive oil have been shown to exert antimicrobial activities against a wide range of pathogens including E. coli, L. monocytogenes, Salmonella enteriditis, and Salmonella Typhi (Medina et al., 2007; Karaosmanoglu et al., 2010; Paudel et al., 2011; Gabriel et al., 2019), suggesting that VOO could be used as a natural alternative for the prevention of food spoilage (Bubonja-Sonje et al., 2011).
Regarding fresh salad, the production of QSB (quick salad bags) has contributed to a massive increase in salad consumption worldwide (Pilone et al., 2017, Losio et al., 2015). QSB are minimally processed foods consumed without the need for additional treatment. Their minimal processing guarantees the preservation of the product’s sensorial features but results in a shorter shelf-life (Oliveira et al., 2010). The high microbiological risk is the main problem associated with the consumption of these products as microbiological contamination is very common in vegetables growing in soil but also for their moisture content, pH (6.0–7.0), transportation, and storage. Indeed, several outbreaks of food-poisoning have been connected to the consumption of salads contaminated with Salmonella spp. (Vestrheim et al., 2016), L. monocytogenes (Stephan et al., 2015), or E. coli O157: H7 (Arienzo et al., 2020).
In this work we evaluated the antibacterial activity of 13 VOOs produced using different national (N, Italian) or local (L, Sardinian) olive varieties harvested in the same area (Oristano, Sardinia, Italy). The oils were tested in vitro against Gram-positive (+) and Gram-negative (-) bacteria (both pathogenic and pro-technological strains). The two oils presenting the best in vitro antimicrobial activities against the pathogenic bacteria L. monocytogenes DSM 20600 and S. bongori DSM13772 were then further tested in the context of commercial baby green leaf lettuce QSB samples for their potential use as antimicrobial ingredients.
The antimicrobial activities of the VOO samples were tested against four pathogenic bacteria. The majority of the VOOs showed strong antimicrobial activity against S. aureus DSM 20231 and L. monocytogenes DSM 20600, whereas weaker activity was observed against S. bongori DSM 13772 and E. coli DSM 30083. Moreover, the VOO level of antimicrobial activity was dependent on both the pathogen strain treated and the olive oil contact time (0 min [T0] vs 1 h [T1]).
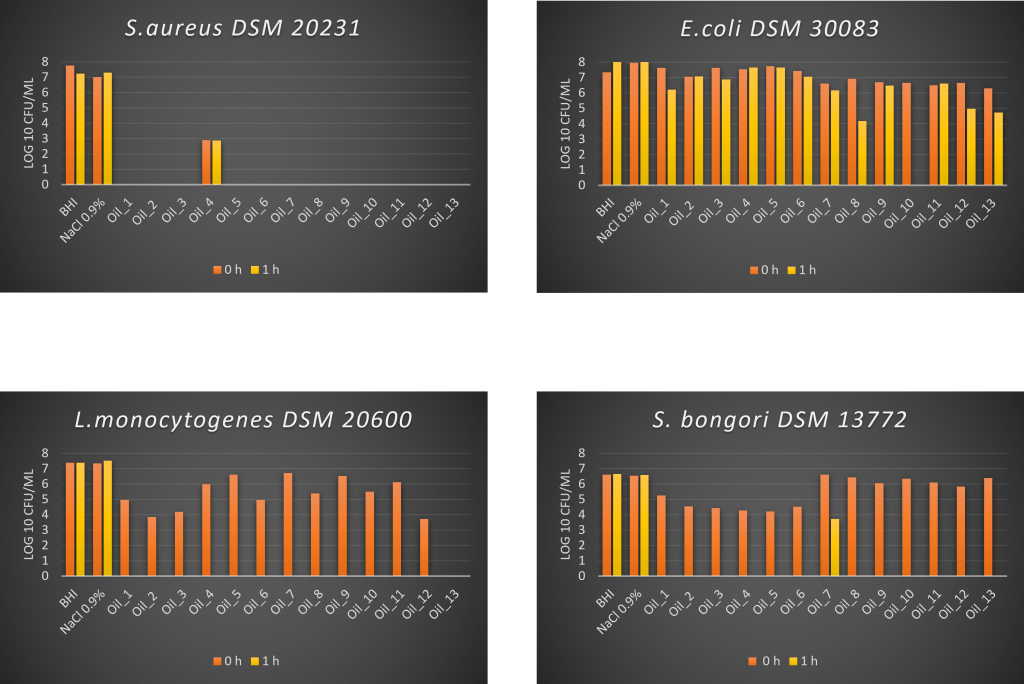
Differences in the inhibition rate between Gram(+) and Gram(-) bacteria due to the external membrane that characterize the Gram(-) groups were also noticed, in agreement with the few other works present in literature (Sofo et al., 2018; Zampa et al., 2010). To this regard, Medina et al. (2006, 2007) found that Gram(-) bacteria, S. sonnei and E. coli, partly survived after one hour of treatment with virgin olive oil treatment, whereas a much stronger bactericidal effect was observed against S. aureus, L. monocytogenes and Yersinia sp. More recently, Hatami et al. (2016) found that an organic VOO was able to inhibit L. innocua, although less activity was observed against E. coli and S. aureus.
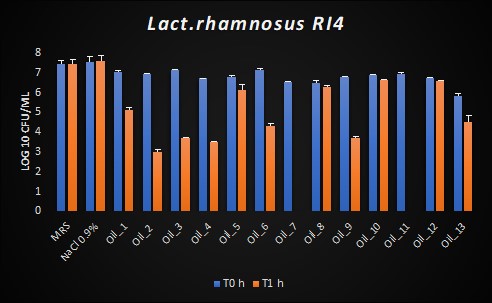
Concerning Lactobacilli, we assessed VOO activity against two commercial probiotics and one strain isolated from olive oil. The resistance of the latter, Lac. rhamnosus RI4, previously isolated from VOO, to the majority of the VOOs tested probably reflects its adaptation to the environment from which it was isolated. The two commercial probiotics studied, Lim. reuteri DSM 17938 and Lac. paracasei Shirota, showed good resistance at T0, but not at T1.
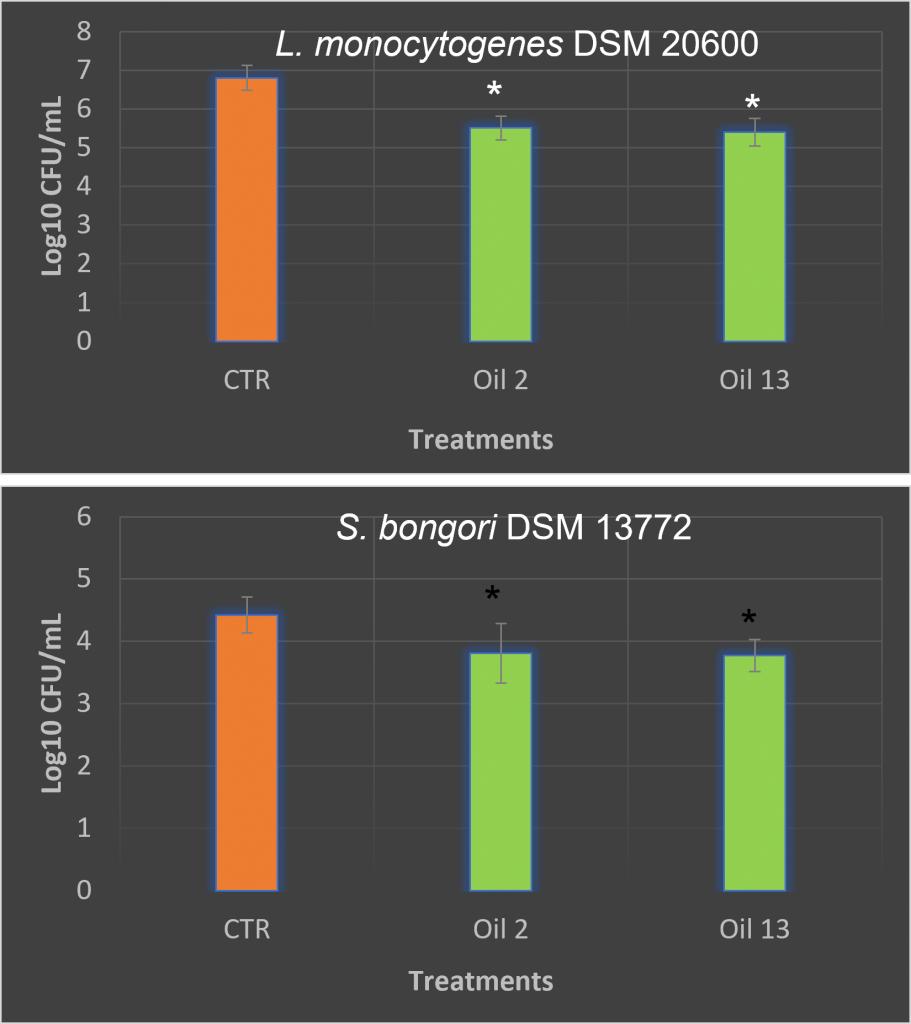
For the use of olive oils as an antimicrobial ingredient in QSB, we tested the two VOOs showing the best antimicrobial activities (Bosana and Sivigliana VOO) against lettuce samples taken from QSB inoculated with the pathogensS. bongori DSM 13772and L. monocytogenes DSM 20600. The two pathogens showed different behaviors in response to the initial VOO concentration, which also depended on the inoculum concentration, while no differences were observed between the antimicrobial activities of the two oils tested. Concerning the inoculum concentration, we found that the higher the initial inoculum concentration, the lower the percentage of attached cells. This is probably because according to Mathew at al., (2018) the attachment of pathogens cells to vegetable surface is time dependent, so the probability of single cells to attach to the surface of lettuce is lower when a high inoculum is used than a lower inoculum, so increasing the competition for available surfaces. Moreover, the higher competition for nutrients may reduce the production of bacterial appendages responsible for the attachment process.
In conclusion, the small number of in vitro studies conducted on the antimicrobial activities of VOOs provide strong evidence in support of the health-benefiting properties of this widely used condiment. The present study investigated in detail, for the first time, the effects of two VOOs used as an antimicrobial in a food matrix to reduce or eliminate foodborne pathogens in minimally processed foods when added to QBS as dressings. Finally, although this work strongly supports the utilization of olive oils, not only for their nutraceutical, but also for their antimicrobial properties, it is important to underline that its utilization could have some limits (also important to be examined closely) mostly due to the organoleptic modifications of olive oils (own or induced) when they are added as dressings if RTEs are not quickly consumed.

Severino Zara Associate Professor in the scientific disciplinary sector of Agricultural Microbiology, he has been in service since October 2007 at the Department of Agriculture of the University of Sassari, Italy. Since the 2008 he has been a lecturer in Microbiology and Genetics of Microorganisms of the degree course in Viticulture Oenology and Food Technologies. The research activity of Prof. Severino Zara since 2007 has been framed both in the research direction followed by the Microbiology Group of the Department of Agriculture and collaborations with other Italian and foreign University Departments. These research lines can be summarized as follows: Study of biofilms in yeast; Interaction of wine yeasts and pesticides; Use of mixed starter microorganisms in fermentation; Interaction of microorganisms and essential oils; Cell wall study in Saccharomyces and non-Saccharomyces wine yeasts; Study of the microflora in olive oils. Since 2009 he has been a member of the board of the PhD Course in Agriculture Sciences and since 2012, he has been Coordinator of the Curriculum in Agri-food Microbial Biotechnology of the above Course. Since 2021 is Coordinator of the Course of Doctorate in Agricultural Sciences. In 2010 he carried out a three-month period of study and research as invited research at the laboratory of Dr. Alan Bakalinsky – Department of Food Science and Technology – Oregon State University, USA, with whom he has continuous collaborative relationships. He is scientific director and participant in research projects funded by RAS (Autonomous Region of Sardinia), MIUR (Italian Ministry of Education, University and Research) and the Foundation of Sardinia. He collaborates with Italian and foreign Universities. Author of more than 100 scientific papers of which 50 in international peer-reviewed journals.
References
- Arienzo, A., Murgia, L., Fraudentali, I., Gallo, V., Angelini, R., Antonini, G. (2020). Microbiological Quality of Ready-to-Eat Leafy Green Salads during Shelf-Life and Home refrigeration. Foods, 9, 1421. https://doi.org/10.3390/foods9101421
- Brenes, M., Medina, E., Romero, C., & de Castro, A. (2007). Antimicrobial activity of olive oil. Agro Food Industry Hi-Tech, 18, 6-8.
- Bubonja-Sonje, M., Giacometti, J., & Abram, M., (2011). Antioxidant and antilisterial activity of olive oil, cocoa and rosemary extract polyphenols. Food Chemistry 127, 1821-1827. https://doi.org/10.1016/j.foodchem.2011.02.071
- D’Angelo, S., Ingrosso, D., Migliardi, V., Sorretino, A., Donnarumma, G., Baroni, A., Masella, L., Tufano, M. A., Zappia, M., & Galletti, P. (2005). Hydroxytyrosol, a natural antioxidant from olive oil, prevents protein damage induced by long-wave ultraviolet radiation in melanoma cells. Free Radical Biology & Medicine, 38, 908 – 919. https://doi.org/10.1016/j.freeradbiomed.2004.12.015
- Gabriel, P. O., Aribisala, J. O., Oladunmoye, M. K., Arogunjo, A. O., & Ajayi-Moses, O. B. (2019). Therapeutic Effect of Goya Extra Virgin Olive Oil in Albino Rat Orogastricallly Dosed with Salmonella Typhi. South Asian Journal of Research in Microbiology, 1-9. DOI: 10.9734/sajrm/2019/v3i230084
- Hatami, S., Sani, A. M., & Yavarmanesh, M. (2016). Chemical composition and antibacterial activity of organic extra virgin olive oil from Iran. Nutrition & Food Science, 46, 388-395. https://doi.org/10.1108/NFS-01-2016-0010
- Karaosmanoglu, H., Soyer, F., Ozen, B., & Tokatli, F. (2010). Antimicrobial and antioxidant activities of Turkish extra virgin olive oils. Journal of agricultural and food chemistry, 58, 8238-8245. https://doi.org/10.1021/jf1012105
- Losio, M.N., Pavoni, E., Bilei, S., Bertasi, B., Bove, D., Capuano, F., Farneti, S., Blasi, G., Comin, D., Cardamone, C., Decastelli, L., Delibato, E., De Santis, P., Di Pasquale, S., Gattuso, A., Goffredo, E., Fadda, A., Pisanu, M., & De Medici, D. (2015). Microbiological survey of raw and ready-to-eat leafy green vegetables marketed in Italy. International Journal of Food Microbiology, 210, 88–91. https://doi.org/10.1016/j.ijfoodmicro.2015.05.026
- Mathew, E. N., Muyyarikkandy, M. S., Kuttappan, D., & Amalaradjou, M. A. (2018). Attachment of Salmonella enterica on Mangoes and Survival Under Conditions Simulating Commercial Mango Packing House and Importer Facility. Frontiers in microbiology, 9, 1519. https://doi.org/10.3389/fmicb.2018.01519
- Medina, E., De Castro, A., Romero, C., & Brenes, M. (2006). Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: correlation with antimicrobial activity. Journal of Agricultural and Food Chemistry, 54, 4954-4961. DOI: 10.1021/jf0602267
- Medina, E., Romero, C., Brenes, M., & De Castro, A. (2007). Antimicrobial activity of olive oil, vinegar, and various beverages against foodborne pathogens. Journal of food protection, 70, 1194-1199. https://doi.org/10.4315/0362-028X-70.5.1194
- Oliveira, M. M., de Almeida, F. A., Baglinière, F., de Oliveira, L. L., & Vanetti, M. C. D. (2021). Behavior of Salmonella Enteritidis and Shigella flexneri during induction and recovery of the viable but nonculturable state. FEMS Microbiology Letters, 368(14).
- Paudel, S., Magrati, T., & Lamichhane, J. R. (2011). Antimicrobial activity of wild olive crude extracts in vitro. International Journal of Pharma Sciences and Research, 2, 110-113.
- Pilone, V., Stasi, A., & Baselice, A. (2017). Quality preferences and pricing of fresh-cut salads in Italy: new evidence from market data. British Food Journal, 119, 1473–1486. https://doi.org/10.1108/BFJ-09-2016-0419
- Sofo, A., Elshafie, H. S., Scopa, A., Mang, S. M., & Camele, I. (2018). Impact of airborne zinc pollution on the antimicrobial activity of olive oil and the microbial metabolic profiles of Zn-contaminated soils in an Italian olive orchard. Journal of Trace Elements in Medicine and Biology, 49, 276-284. https://doi.org/10.1016/j.jtemb.2018.02.017
- Stephan, R., Althaus, D., Kiefer, S., Lehner, A., Hatz, C., Schmutz, C., Jost, M., Gerber, N., Baumgartner, A., Hachler, H., & Mäusezahl-Feuz, M. (2015). Foodborne transmission of Listeria monocytogenes via ready-to-eat salad: A nationwide outbreak in Switzerland, 2013–2014. Food control, 57, 14-17. https://doi.org/10.1016/j.foodcont.2015.03.034
- Vestrheim, D. F., Lange, H., Nygård, K., Borgen, K., Wester, A. L., Kvarme, M. L., & Vold, L. (2016). Are ready-to-eat salads ready to eat? An outbreak of Salmonella Coeln linked to imported, mixed, pre-washed and bagged salad, Norway, November 2013. Epidemiology & Infection, 144, 1756-1760. doi:10.1017/S0950268815002769
- Visioli, F., Caruso, D., Grande, S., Bosisio, R., Villa, M., Galli, G., Sirtori, C., & Galli, C. (2005). Virgin Olive Oil Study (VOLOS): vasoprotective potential of extra virgin olive oil in mildly dyslipidemic patients. European Journal of Nutrition, 44, 121-127. DOI 10.1007/s00394-004-0504-0
- Yakhlef, W., Arhab, R., Romero, C., Brenes, M., de Castro, A., & Medina, E. (2018). Phenolic composition and antimicrobial activity of Algerian olive products and by-products. LWT, 93, 323-328. https://doi.org/10.1016/j.lwt.2018.03.044
- Zampa, A., Silvi, S., Servili, M., Montedoro, G., Orpianesi, C., & Cresci, A. (2006). In vitro modulatory effects of colonic microflora by olive oil iridoids. Microbial ecology in health and disease, 18, 147-153. https://doi.org/10.1080/08910600600994940

