By Julio Sánchez Poblete
During wine production and storage processes, the presence of metal ions must be controlled, since an excessive concentration considerably affects the preservation and quality of the product, either in the alteration of sensory parameters or even causing health problems for the consumer1. Because metal ions when present in concentrations even at trace levels cause oxidation-reduction reactions that affect the organoleptic characteristics, such as colour, aroma, astringency and mouthfeel2. Therefore, elements and compounds involved in the destabilization of wines and in their oxidative evolution, such as oxygen, polyphenols, iron, copper, and manganese metal ions, should be routinely monitored2,3,4. In this context, the ferric cation (Fe3+) is the metal element most likely to produce alterations in wine because it is the most common. At concentrations above 10 mg L-1 it forms an insoluble suspension known as “ferric case”5. Therefore, the control of these metal ions is of utmost importance during winemaking and wine preservation processes due to their effects on subsequent consumption6.
Currently, several metal ion remediation methods are applied in the wine industry, with the potassium ferrocyanide method, also called “blue clarification”, being the most widely used7. However, this method has drawbacks, such as, limited efficacy and the generation of wastewater requiring complicated treatment8. Therefore, alternative methods that can remove metal ions present in wines without altering their organoleptic characteristics have been studied, such as extraction procedures to separate metal ions, which are generally based on ion exchange resins9; liquid-liquid; microextraction10; cloud point removal11; and solid-phase extraction12. Among these techniques, ion exchange treatments have received considerable scientific attention because the resins have achieved iron removal efficiencies close to 100% without generating sludge13, and it is also a more economical technique than “blue clarification” in wines.
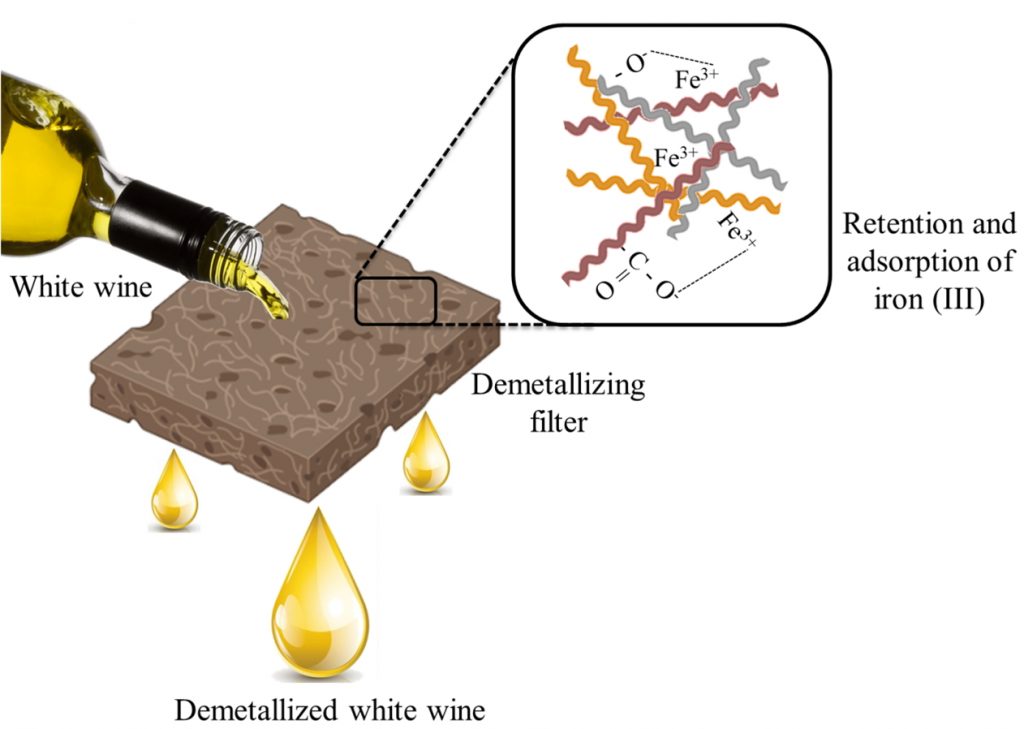
The use of bio-based polymeric materials reinforced with cellulose and capable of removing metal ions in aqueous solution have attracted scientific attention due to being a biodegradable, renewable and abundant material14. Fibrillated cellulose (CF) has been previously used to remove chromium in hydrogels and has mechanical reinforcement characteristics15. While plant fibres, such as Scirpus californicus (SC) and Gunnera tinctoria (GT) have attracted scientific attention. SC fibres are mainly found in lakes and can grow in environments containing toxic pollutants. On the other hand, GT is a non-timber forest product, of recognized importance in southern Chile, mainly at the level of small producers, where individuals and/or rural groups are dedicated to its collection and informal sale. Based on the above, this research presents an effective and practical alternative for iron removal in aqueous solutions and organic white wines, based on continuous filtration through mechanically prepared filters, composed of vegetable fibres of SC, GT, and CF, extracted from Eucalyptus globulus (Eucalyptus) (see Figure 1). These filter materials were characterized by techniques such as Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), thermogravimetric analysis (TGA) and dynamic mechanical analysis (DMA). FT-IR signals confirmed that hydroxyl and carboxyl groups were present in the fibres; SEM micrographs showed a compact surface morphology among the three fibres studied; TGA and DMA identified a thermal enhancement and an increase in the compressive strength of the blend after CF addition. In addition, the effect of the amount of each fibre on iron retention in water and wine was evaluated, where the results showed that filters with 15% CF presented better iron retention performance. In addition, the iron retention capacities or efficiencies were 139.4 mg kg-1, 57.3% in aqueous solution and 26% in white wine, using the system composed of 21% GT, 64% SC and 15% CF. These results indicate the potential of these natural fibres in the demetallization of organic wines.
See the original article for detailed information on this research published in the scientific Journal Environmental Technology & Innovation:
José Neira, Andrés Boulett, Karina Roa, Diego Oyarzún, Julio Sánchez. “Vegetable filters reinforced with fibrillated cellulose for iron removal from water and organic white wines”. Environmental Technology & Innovation. 25 (2022) 102104. https://doi.org/10.1016/j.eti.2021.102104.

Dr. Julio Antonio Sánchez Poblete
Julio Sánchez Poblete obtained his Ph.D. degree in Chemistry (2010) from the Universidad de Concepción (Concepción, Chile) and his Ph.D. degree in Chemistry, Specialty in Polymer Science (2010) from the Université Joseph Fourier (Grenoble, France). He completed a postdoctoral training (2015) at University of Helsinki, Finland, which focused on polymeric materials applied in Cr(VI) removal. Currently, he is an associate professor at the Department of Environmental Sciences of the Faculty of Chemistry and Biology of the University of Santiago de Chile (USACH) and director of the Laboratory of Advanced Materials for Environmental Chemistry of the USACH (Santiago, Chile). Dr. Julio Sánchez has published more than 85 indexed articles (Scopus), 8 book chapters, and his research interests are focused on developing new advanced, efficient, reusable and environmentally friendly materials, such as: hydrogels, water soluble polymers and interpolymeric membranes, for the removal of contaminants such as: dyes, antibiotics, heavy metals, and arsenic in aqueous solution, by means of adsorption methods, polymer-assisted ultrafiltration membranes, and hybrid methods.
References
- Čepo, et al., Food Chem. 246 (2018) 394-403. 10.1016/j.foodchem.2017.10.133
- Kontoudakis, et al., Food Chem. 274 (2019) 88-99. 10.1016/j.foodchem.2018.08.084
- Peña, et al., Fermentation. 5 (2019) 1-25. 10.3390/fermentation5010025
- Tariba, et al., Biol. Trace. Elem. Res. 144 (2011) 143-156, 10.1007/s12011-011-9052-7
- Vallejos, et al., J. Mater. Chem. A. 1 (2013) 15435-15441. 10.1039/C3TA12703F
- Lan, et al., Food Chem. 345 (2021) 128854. 10.1016/j.foodchem.2020.128854
- Mitreva, et al., Microchem. J. 132 (2017) 238-244. 10.1016/j.microc.2017.01.023
- Palacios, et al., Adsorption. 7 (2001) 131-138. 10.1023/A:1011600207978
- Chen, et al., Anal. Sci. 28 (2012) 795-799. 10.2116/analsci.28.795
- Chamsaz, et al., Int. J. Environ. Anal. Chem. 95 (2014) 345-348. 10.1080/03067319.2013.840831
- Filik, et al., Food Chem. 130 (2012) 209-213. 10.1016/j.foodchem.2011.07.008
- Miranda, et al., Talanta. 148 (2016) 633-640. 10.1016/j.talanta.2015.05.062
- Martins, et al., J. Mol. Liq. 242 (2017) 505-511. 10.1016/j.molliq.2017.07.041
- Han, et al., ACS Sustain. Chem. Eng. 4 (2016) 5667-5673. 10.1021/acssuschemeng.6b01567
- Dax, et al., Nord. Pulp Paper Res. J. 30 (2015) 373-384. 10.3183/npprj-2015-30-02-p369-372