By Pierluigi Plastina
Olive oil (OO) is a hallmark of the Mediterranean diet as it represents its main source of fat. Its worldwide consumption has increased during the last five years (1) and this success relies on its organoleptic properties as well as on its nutritional value and its health promoting effects. Olive oil is characterized by a favorable nutrient composition, including monounsaturated oleic acid (OA) as the most abundant fatty acid and fat-soluble vitamins, and by the presence of minor components such as phenolic compounds (2). These latter, also known as polyphenols, have been recognized to contribute to the positive health effects related to the consumption of extra virgin olive oil (EVOO) (3). Tyrosol and hydroxytyrosol (Ty and Hty, respectively) are among the main phenolic compounds found in olive and olive oil. They occur in their free form as well as in their esterified forms, mainly as secoiridoid derivatives (ligstroside and oleuropein, respectively) (4).

During the last few years, our research group has investigated on the occurrence and properties of lipophenols, an emerging subclass of phenolic compounds characterized by the presence of a lipid moiety. These compounds have been largely considered as semi-sinthetic derivatives with enhanced solubility in lipid systems and higher metabolic stability with respect to their corresponding hydrophilic phenolics (5). We hypothesized that tyrosyl oleate and hydroxytyrosyl oleate (TyOle and HtyOle, respectively) could occur in olives and in OO as a result of the reaction between tyrosol (or hydroxytyrosol) and oleic acid, all occurring in relatively high amount.
Indeed, we identifified and quantified both compounds in EVOO (6,7), as well as in the by-products of olive oil industry, pomace and olive mill waste waters (OMWWs) (8). On the other hand, we did not detect TyOle nor HtyOle in olive fruits. These molecules are likely formed as a result of an esterification reaction catalyzed by an appropriate enzyme activated by the pressing process during OO production. Interestingly, HtyOle was found to be more abundant in EVOO than OO (9), while TyOle was found in higher levels in low-quality and defective oils than in EVOOs (6). This could suggest that HtyOle could potentially represent a marker of quality of the oil. By contrary, the presence of TyOle is likely correlated to the increase in the defect and to the increase in the concentration of precursors both during the rancidity, crushing and kneading processes.
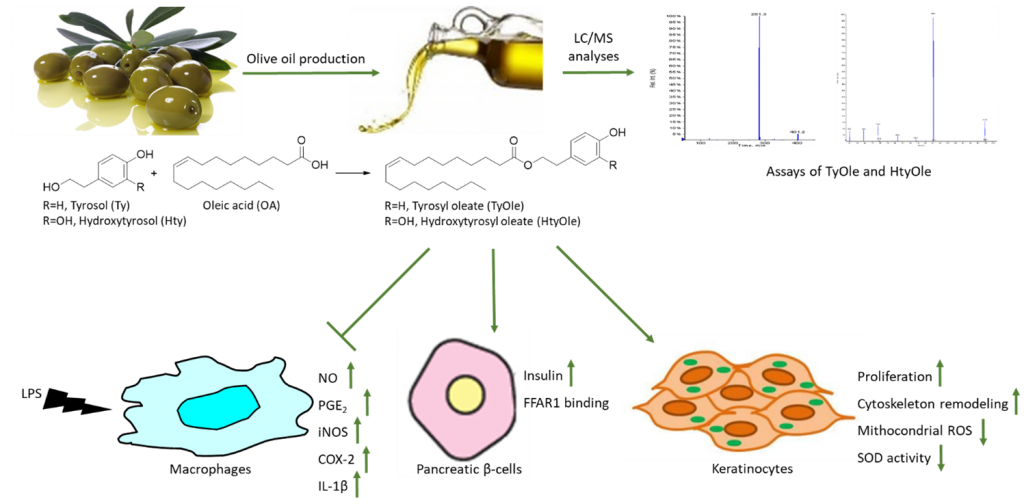
We found that HtyOle possesses in vitro anti-inflammatory properties in macrophages, while both TyOle and Hty were ineffective in our model (8). In particular, HtyOle inhibited lipopolysaccharide (LPS)-induced production of nitric oxide (NO) and prostaglandin E2 (PGE2). At a transcriptional level, HtyOle inhibited the expression of pro-inflammatory genes, including inducible NO synthase (iNOS), cyclooxygenase-2 (COX-2) and interleukin-1β (IL-1β). The association between inflammation and type 2 diabetes and the idea that anti-inflammatory compounds might be potential antidiabetic leads prompted us to investigate the ability of lipophenols to modulate insulin secretion in vitro. We found that both lipophenols exerted insulin secretagogue activity in pancreatic β-cell line under glucose-induced insulin secretion (GSIS) condition. Moreover, through functional pharmacological tests and molecular docking study simulation, we provided evidence that both compounds are natural modulators of free fatty acid receptor 1 (FFAR1), also known as G-protein-coupled receptor 40 (GPR40) (10). Proliferative and antioxidant properties of TyOle and HtyOle were investigated in human keratinocyte cell line. We found that TyOle and HtyOle displayed antioxidant activity by reducing the production of mitochondrial reactive oxygen species (ROS) and the enzymatic activity of superoxide dismutase (SOD). Both lipophenols showed tissue regenerating properties and could represent a valuable adjuvant against skin oxidation and aging (6,7).
Conclusions: Taken together, our results suggest that olive lipophenols represent a significant form in which tyrosol and hydroxytyrosol occur. Conjugation with oleic acid moiety boosts the properties of Ty and Hty as the corresponding lipophenols displayed enhanced anti-inflammatory, antidiabetic, antioxidant and tissue-regenerating properties with respect to the simple phenolics.
Read more at: https://www.mdpi.com/2076-3921/10/7/1051


Pierluigi Plastina obtained his degree in Chemistry in 2002 and his Ph.D. in Organic Chemistry in 2005 from University of Calabria (Rende, Italy). He was research fellow at the Department of Chemistry of the same University in 2006-2007 working on the development of new methods for the quantitative analysis of bioactive compounds in food matrices by means mass spectrometric techniques. He was post-doctoral fellow at the Division of Human Nutrition of the Wageningen University (Wageningen, The Netherlands) in 2007-2008 working under the supervision of Professor Renger Witkamp on n-3 fatty acid ethanolamides with anti-inflammatory properties. Then he moved back to University of Calabria as a research fellow working on the extraction, identification and pharmacological characterization of bioactive compounds from food matrices. He was visiting scientist at the Wageningen University in 2012 and again in 2013. He is currently permanent senior researcher and adjunct professor of Food Chemistry at the Department of Pharmacy, Health and Nutrition Sciences of the University of Calabria. His main research interests are devoted to the investigation of bioactive lipids with potential anti-inflammatory, antioxidant, anticancer and antidiabetic properties and their identification in food matrices and waste materials.
References
- European Commission. Available online: https://ec.europa.eu/info/food-farming-fisheries/plants-and-plant-products/plant-products/olive-oil (accessed on 25 May 2021).
- Visioli, F.; Davalos, A.; López de Las Hazas, M.C.; Crespo, M.C.; Tomé-Carneiro, J. An overview of the pharmacology of olive oil and its active ingredients. Br. J. Pharmacol. 2020, 177, 1316–1330.
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). EFSA J. 2011, 9, 2033.
- Cicerale, S.; Conlan, X.A.; Sinclair, A.J.; Keast, R.S. Chemistry and health of olive oil phenolics. Crit. Rev. Food Sci. Nutr. 2009, 49, 218–236.
- Bayrasy, C.; Chabi, B.; Laguerre, M.; Lecomte, J.; Jublanc, E.; Villeneuve, P.; Wrutniak-Cabello, C.; Cabello, G. Boosting antioxidants by lipophilization: A strategy to increase cell uptake and target mitochondria. Pharm. Res. 2013, 30, 1979–1989.
- Benincasa, C.; La Torre, C.; Fazio, A.; Perri, E.; Caroleo, M.C.; Plastina, P., Cione, E. Identification of tyrosyl oleate as a novel olive oil lipophenol with proliferative and antioxidant properties in human keratinocytes. Antioxidants 2021, 10, 1051.
- Benincasa, C.; La Torre, C.; Plastina, P.; Fazio, A.; Perri, E.; Caroleo, M.C.; Gallelli, L.; Cannataro, R.; Cione, E. Hydroxytyrosyl Oleate: Improved Extraction Procedure from Olive Oil and By-Products, and In Vitro Antioxidant and Skin Regenerative Properties. Antioxidants 2019, 8, 233.
- Plastina, P.; Benincasa, C.; Perri, E.; Fazio, A.; Augimeri, G.; Poland, M.; Witkamp, R.; Meijerink, J. Identification of hydroxytyrosyl oleate, a derivative of hydroxytyrosol with anti-inflammatory properties, in olive oil by-products. Food Chem. 2019, 279, 105–113.
- Lee, Y.Y.; Crauste, C.; Wang, H.; Leung, H.H.; Vercauteren, J.; Galano, J.M.; Oger, C.; Durand, T.; Wan, J.M.; Lee, J.C. Extra Virgin Olive Oil Reduced Polyunsaturated Fatty Acid and Cholesterol Oxidation in Rodent Liver: Is This Accounted for Hydroxytyrosol-Fatty Acid Conjugation? Chem. Res. Toxicol. 2016, 29, 1689–1698.
- Caroleo, M.C.; Plastina, P.; Fazio, A.; La Torre, C.; Manetti, F.; Cione, E. Olive oil lipophenols induce insulin secretion in 832/13 beta cell models. Pharmaceutics 2021, 13, 1085.

