By Astrid Buica and Gonzalo Garrido-Bañuelos
Phenolics have been and continue to be one of the most active fields in grape and wine research. Due to their varied chemical structures, phenolics belong to different classes and show reactivity towards members of the same class, other phenolics classes, and many other grape and wine components. As the medium in which phenolics are present is also changing (physically and chemically) due to the winemaking process, even more opportunities for chemical change arise.
One of the grape berry components that has demonstrated activity towards phenolics is the cell wall material, especially the polysaccharides. A research field in its own rights, polysaccharides also show complexity of chemical structure and reactivity.
The interest in both phenolics and cell wall (poly)saccharides has been constantly increasing; this is possibly due to the development of new analytical methods, but also to the realisation that relevant interactions between these two types of compounds go beyond grape and must, reaching as far as wine aging and sensory aspects. Our recent review1 covers the literature published in the past ten years, including research that has focused on a specific winemaking stage and works that evaluated the interactions between selected classes of phenolics (anthocyanins and condensed tannins or proanthocyanidins) throughout winemaking. To facilitate the navigation through the stages (from vineyard to glass), we have included Figure 1 summarising factors, effects, and interactions described in the literature between the classes of interest.
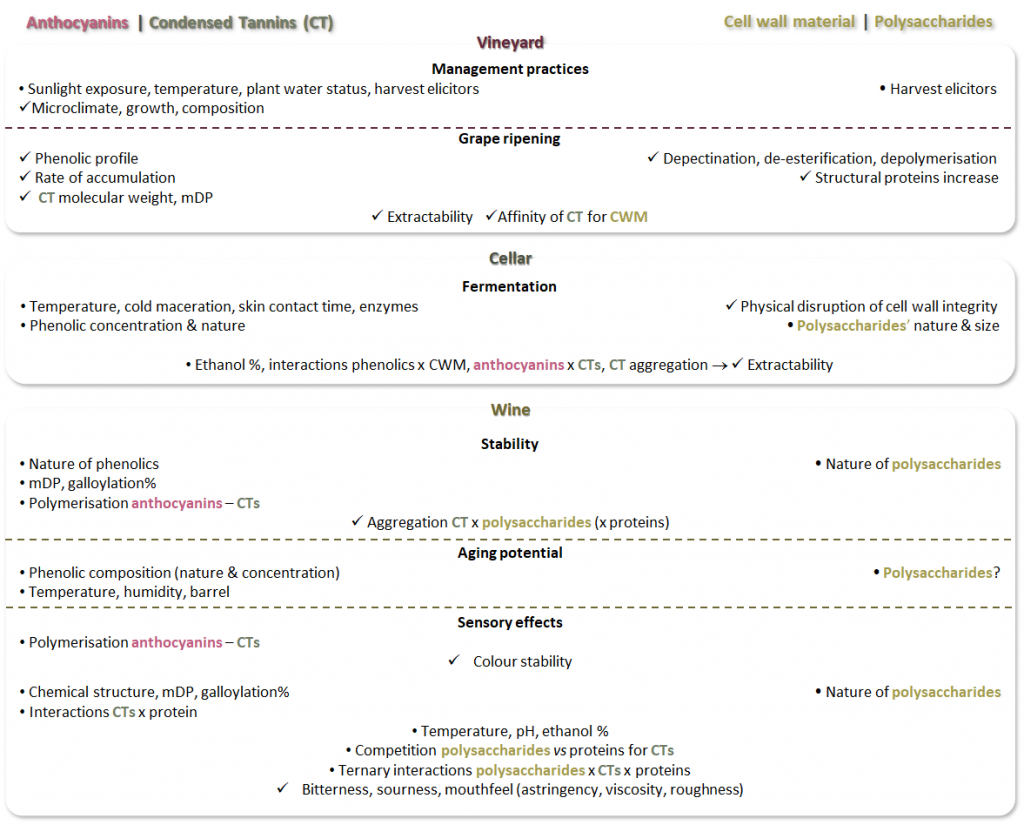
Figure 1. Overview of factors “●” and effects “✓”, including interactions (x), evaluated in the literature review. The aspects mentioned to either side of the figure refer to phenolics on the left, cell wall material on the right, and common aspect in the center.
In the grape, phenolics can be found free or bound to cell wall polysaccharides. Most of the literature to date focuses on the changes both classes go through during ripening and how vineyard management affects accumulation, degradation, and the nature of the composition. The interactions present between phenolics and polysaccharides in the grape are non-covalent – for example hydrogen bonds and hydrophobic interactions2,3. As the degree of ripeness of the grape can affect the cell wall framework (as for example the skin cell wall porosity) and also composition and chemical structure of the phenolics and cell wall material, it will also have an indirect effect on their relationship. Climatic differences between vintages can also influence the cell wall composition and the berry cell wall de-pectination pattern during ripening4. The presence of covalent bonds is still to be demonstrated in grapes.
The structural disruption happening during crushing brings all phenolics and polysaccharides in direct contact. Once more, the external factors associated with winemaking (such as addition of maceration enzymes, temperature, skin contact time, etc.) can influence the extent of the interactions5–8. Effects deriving from the berry (as affected by ripeness for example) will also contribute: cell wall structural integrity, composition of the cell wall material and phenolics, protein content, etc. At this stage in the winemaking, new interactions appear which were not possible when the compounds were physically separated in the structurally intact berry. Phenolics associate with soluble and insoluble cell wall material, with other classes of phenolics, or with compounds within the same phenolic class9. Competition between the two classes of phenolics (anthocyanins and tannins) for the active sites on the cell wall material is also possible10. Associations and adsorption phenomena can be however restricted not only by the chemical nature of the molecules (for example degree of galloylation11), but also by molecule size, as is the case of tannins12. A lot of work has been dedicated to the effect of practical winemaking procedures (such as the use of enzymes13–15 and yeast selection16–18) that can influence the extraction of phenolics, cell wall polysaccharide solubilisation, and subsequently wine composition.
The effect of polysaccharides has been less investigated in wine, especially from the perspective of wine aging potential and of sensory effects. They appear to have an effect on the colour stability19 and their association with anthocyanins is influenced by the ethanol level3. The nature of the polysaccharides20 and the temperature also play a role in the stability of the complexes formed21. These interactions have led to the utilisation of fermented grape as more natural and sustainable fining agent for the wine industry22.
The role phenolics play in the taste and mouthfeel of a wine has been a research topic for a number of years, resulting in the (partial) elucidation of the factors playing a role (mDP, degree of galloylation, etc.) and in the detailed description of the sensations produced (‘puckering’, ‘chalky’, ‘dryness’, ‘fullness’, etc.). Recent work also demonstrated that polysaccharides contribute to in-mouth perception23 of red wines. The interactions taking place are complex and can competitively include anthocyanins24,25, tannins, polysaccharides, and salivary proteins25–27. These interactions are believed to contribute to the “subqualities” of astringency perception. Factors such as temperature, pH, and ethanol concentration also affect the in-mouth perception of wines.
The relationship between phenolics and grape polysaccharides is undoubtedly complex; many other factors and compounds present in the matrix add new layers of difficulty in understanding the interactions at a theoretical level and translating them into practical advice for the winemakers. The sensory aspect is also of practical significance as it includes inherent variability of the consumers. With the development of new analysis methods, the field of polysaccharides x phenolics (x proteins) will be active for a number of years to come.
Read more at: https://www.tandfonline.com/doi/abs/10.1080/10408398.2021.1918056?journalCode=bfsn20

Astrid Buica 
Gonzalo Garrido-Bañuelos 
Astrid Buica is a researcher in Oenology specialized in Analytical Chemistry applied to wine and related matrices, coordinator of Analytical activities and manager of the Analytical Laboratory in the Department of Viticulture and Oenology at the Stellenbosch University, South Africa. Dr Buica dedicates most of her time to the Analytical activities and laboratory in the Department, developing methods for routine analysis of wine and related matrices. In the recent years, she has shifted her focus to include Sensory Science in her work, to bring together these two fields relevant to Wine Sciences. Most of her projects have an important Sensory and Analytical component to them, in an effort to give a more comprehensive view to the evaluation of wine. As such, she delved into untargeted analyses /information-rich techniques and rapid sensory methods for rapid wine profiling, and into the fields of Chemometrics and Data Fusion.
Gonzalo Garrido-Bañuelos. Originally from Logroño (La Rioja), Spain, I completed my studies in Biology (Universidad de Salamanca) in 2011. My contact with wine research started when I moved abroad. I completed my MSc (Université de Bordeaux, France) and my PhD in Oenology (Stellenbosch University, South Africa). During these years, my research has been focusing on bringing together different aspects of wine, from wine chemistry (volatile and phenolic compounds) to cell wall analysis and sensory science. In 2018, after completing my PhD, I moved to Gothenburg, Sweden, to join the Department of Product Design and Perception at RISE – Research Institutes of Sweden. Since then, I have been working on several multidisciplinary research projects (linking food composition and structure properties to sensory) for academia and as well as developing new products for industry.
References
- Garrido-Bañuelos G, Buica A, Toit W. Relationship between anthocyanins , proanthocyanidins , and cell wall polysaccharides in grapes and red wines . A current state-of-art review. Crit Rev Food Sci Nutr. 2021;0(0):1-17. doi:10.1080/10408398.2021.1918056
- Renard CMGC, Watrelot AA, Le Bourvellec C. Interactions between polyphenols and polysaccharides: Mechanisms and consequences in food processing and digestion. Trends Food Sci Technol. 2017;60:43-51. doi:10.1016/j.tifs.2016.10.022
- Medina-Plaza C, Beaver JW, Miller K V, et al. Cell Wall – Anthocyanin Interactions during Red Wine Fermentation-Like Conditions. Am J Enol Vitic. 2020;2:149-156. doi:10.5344/ajev.2019.19063
- Garrido-Bañuelos G, Buica A, Schückel J, et al. Investigating the relationship between grape cell wall polysaccharide composition and the extractability of phenolic compounds into Shiraz wines. Part I: Vintage and ripeness effects. Food Chem. 2019;278(October):36-46. doi:10.1016/j.foodchem.2018.10.134
- Apolinar-Valiente R, Romero-Cascales I, Williams P, et al. Effect of winemaking techniques on polysaccharide composition of Cabernet Sauvignon, Syrah and Monastrell red wines. Aust J Grape Wine Res. Published online 2014. doi:10.1111/ajgw.12048
- Lerno L, Reichwager M, Ponangi R, Hearne L, Block DE, Oberholster A. Effects of cap and overall fermentation temperature on phenolic extraction in cabernet sauvignon fermentations. Am J Enol Vitic. 2015;66(4):444-453. doi:10.5344/ajev.2015.14129
- Martínez-Lapuente L, Guadalupe Z, Ayestarán B. Properties of Wine Polysaccharides. In: Intech Open. Vol i. ; 2019:13. doi:http://dx.doi.org/10.5772/57353
- Holzapfel BP, Watt J, Smith JP, Šuklje K, Rogiers SY. Effects of timing of N application and water constraints on N accumulation and juice amino N concentration in “Chardonnay” grapevines. Vitis – J Grapevine Res. 2015;54(4):203-211. doi:10.5073/vitis.2015.54.203-211
- Castro-López R, Gómez-Plaza E, Ortega-Regules A, Lozada D, Bautista-Ortín AB. Role of cell wall deconstructing enzymes in the proanthocyanidin – cell wall adsorption – desorption phenomena. Food Chem. 2016;196:526-532. doi:10.1016/j.foodchem.2015.09.080
- Kilmister RL, Mazza M, Baker NK, Faulkner P, Downey MO. A role for anthocyanin in determining wine tannin concentration in Shiraz. Food Chem. 2014;152:475-482. doi:10.1016/j.foodchem.2013.12.007
- Bautista-Ortín AB, Abdallah R Ben, Castro-López R, Jiménez-Martínez MD, Gómez-Plaza E. Technological Implications of Modifying the Extent of Cell Wall-Proanthocyanidin Interactions Using Enzymes. Int J Mol Sci Sci. 2016;17:1-12. doi:10.3390/ijms17010123
- Hanlin RL, Hrmova M, Harbertson JF, Downey MO. Review: Condensed tannin and grape cell wall interactions and their impact on tannin extractability into wine. Aust J Grape Wine Res. 2010;16(1):173-188. doi:10.1111/j.1755-0238.2009.00068.x
- Río Segade S, Pace C, Torchio F, Giacosa S, Gerbi V, Rolle L. Impact of maceration enzymes on skin softening and relationship with anthocyanin extraction in wine grapes with different anthocyanin profiles. Food Res Int. 2015;71:50-57. doi:10.1016/j.foodres.2015.02.012
- Gao Y, Fangel JU, Willats WGT, Vivier MA, Moore JP. Dissecting the polysaccharide-rich grape cell wall changes during winemaking using combined high-throughput and fractionation methods. Carbohydr Polym. 2015;133:567-577. doi:10.1016/j.carbpol.2015.07.026
- Zietsman AJJ, Moore JP, Fangel JU, Willats WGT, Trygg J, Vivier MA. Following the Compositional Changes of Fresh Grape Skin Cell Walls during the Fermentation Process in the Presence and Absence of Maceration Enzymes. J Agric Food Chem. 2015;63:2798–2810. doi:10.1021/jf505200m
- Morata A, Loira I, María J, et al. Yeast influence on the formation of stable pigments in red winemaking. Food Chem. 2016;197:686-691. doi:10.1016/j.foodchem.2015.11.026
- Morata A, Escott C, Loira I, Manuel Del Fresno J, González C, Suárez-Lepe JA. Influence of Saccharomyces and non-Saccharomyces yeasts in the formation of pyranoanthocyanins and polymeric pigments during red wine making. Molecules. 2019;24(24). doi:10.3390/molecules24244490
- Caridi A, De Bruno A, De Salvo E, Piscopo A, Poiana M, Sidari R. Selected yeasts to enhance phenolic content and quality in red wine from low pigmented grapes. Eur Food Res Technol. 2017;243(3):367-378. doi:10.1007/s00217-016-2750-9
- Alcalde-Eon C, García-Estévez I, Puente V, Rivas-Gonzalo JC, Escribano-Bailón MT. Color stabilization of red wines. A chemical and colloidal approach. J Agric Food Chem. 2014;62(29):6984-6994. doi:10.1021/jf4055825
- Gonçalves FJ, Fernandes PAR, Wessel DF, Cardoso SM, Rocha SM, Coimbra MA. Interaction of wine mannoproteins and arabinogalactans with anthocyanins. Food Chem. 2018;243:1-10. doi:10.1016/j.foodchem.2017.09.097
- Fernandes A, Brandão E, Raposo F, et al. Impact of grape pectic polysaccharides on anthocyanins thermostability. Carbohydr Polym. 2020;239(April):116240. doi:10.1016/j.carbpol.2020.116240
- Jiménez-Martínez MD, Bautista-Ortín AB, Gil-Muñoz R, Gómez-Plaza E. Fining with purified grape pomace. Effect of dose, contact time and varietal origin on the final wine phenolic composition. Food Chem. 2019;271(May 2018):570-576. doi:10.1016/j.foodchem.2018.08.009
- Chong HH, Cleary MT, Dokoozlian N, Ford CM, Fincher GB. Soluble cell wall carbohydrates and their relationship with sensory attributes in Cabernet Sauvignon wine. Food Chem. 2019;298(May). doi:10.1016/j.foodchem.2019.05.020
- Sáenz-Navajas MP, Avizcuri JM, Ferrero-del-Teso S, Valentin D, Ferreira V, Fernández-Zurbano P. Chemo-sensory characterization of fractions driving different mouthfeel properties in red wines. Food Res Int. 2017;94:54-64. doi:10.1016/j.foodres.2017.02.002
- Soares S, Brandão E, Mateus N, de Freitas V. Sensorial properties of red wine polyphenols: Astringency and bitterness. Crit Rev Food Sci Nutr. 2017;57(5):937-948. doi:10.1080/10408398.2014.946468
- Soares S, Mateus N, De Freitas V. Carbohydrates inhibit salivary proteins precipitation by condensed tannins. J Agric Food Chem. 2012;60(15):3966-3972. doi:10.1021/jf3002747
- Ramos-Pineda AM, Carpenter GH, Garciá-Estévez I, Escribano-Bailón MT. Influence of Chemical Species on Polyphenol-Protein Interactions Related to Wine Astringency. J Agric Food Chem. 2020;68(10):2948-2954. doi:10.1021/acs.jafc.9b00527