By Guilherme Henrique Souza Bomfim
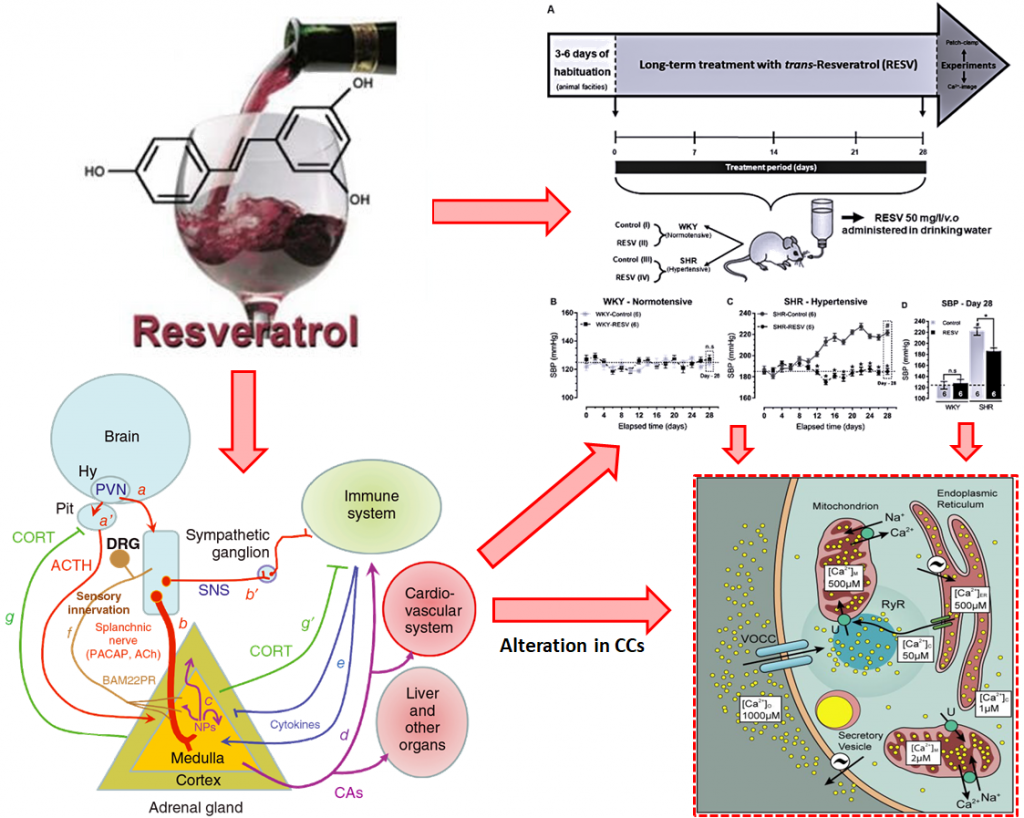
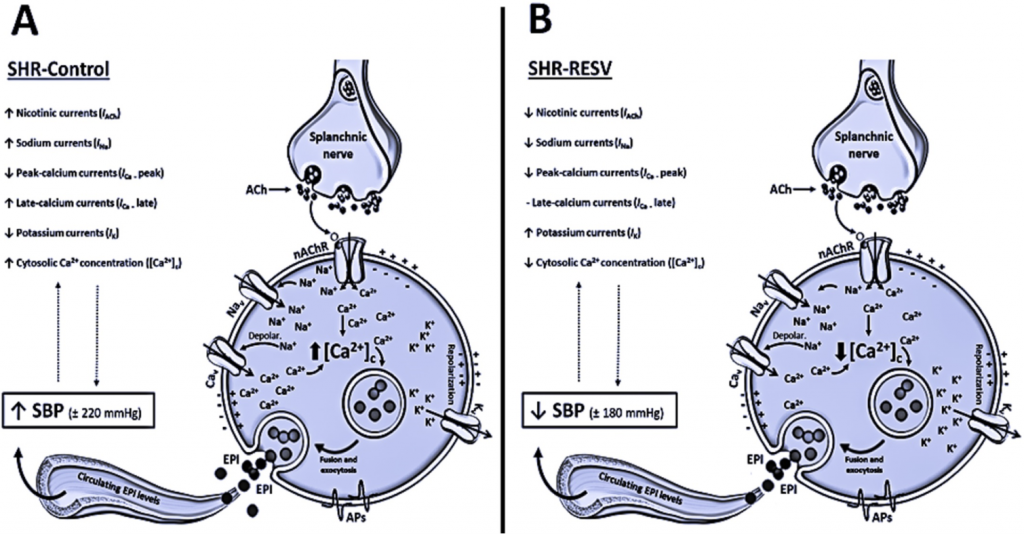
Resveratrol (RESV) is one of the most abundant polyphenol-stilbene compounds found in red wine with well-established cardioprotective and antihypertensive effects. Hyperactivity of the sympathoadrenal axis seems to be one of the major contributing factors in the pathogenesis of human essential hypertension. Alterations in outward voltage-dependent potassium currents (IK) and inward voltage-dependent sodium (INa), calcium (ICa) and nicotinic (IACh) currents, chromaffin cells (CCs) excitability, Ca2+ homeostasis, and catecholamine exocytoses were previously related to the hypertensive state. This raised the issue of whether in vivo long-term RESV treatment can directly act as a modulator of Ca2+ influx or a regulator of ion channel permeability in CCs. We monitored outward and inward currents and cytosolic Ca2+ concentrations ([Ca2+]c) using different pharmacological approaches in CCs from normotensive (WKY) and hypertensive (SHR) animals chronically exposed to trans-RESV (50 mg/L/v.o, 28 days). As we illustrated in Figures 1 and 2, the long-term RESV treatment prevented the increase of the systolic blood pressure (SBP) in SHR, without reversion of cardiac hypertrophy. We also demonstrated that improvement of the outward IK, reduction in inward INa, ICa, and IACh, and the prevention of [Ca2+]c overload are possible new targets in CCs related to the antihypertensive effects of long-term RESV treatment. These findings gain clinical relevance when considering that dietary supplementation or chronic consumption of a moderate amount of red wine has an antihypertensive effect in SHR animals by modulating sympathoadrenal axis functionality.
Read more at: https://www.sciencedirect.com/science/article/abs/pii/S0898656820302886

Department of Molecular Pathobiology, New York University College of Dentistry (NYU), New York-USA.
Corresponding author: Department of Molecular Pathobiology, New York University – College of Dentistry, New York, NY 10010, USA. E-mail addresses: ghs5@nyu.edu; henrique_epm@hotmail.com
Guilherme Henrique Souza Bomfim received his MSc and Ph.D. in Pharmacology at the Federal University of São Paulo (Brazil). During his Ph.D. studies he also spent time at the Universidad Autónoma de Madrid (Spain), focusing on mechanisms of action and channelopathies involved in calcium (Ca2+) signaling using electrophysiological (Patch-Clamp and Amperometry), fluorescence (Ca2+ dyes), and molecular (siRNA and plasmid DNA) techniques.
Currently, in the New York University, I am focused on the molecular modulators of the Ca2+ release-activated Ca2+ (CRAC) channel, also known as Store-operated Ca2+ Entry (SOCE). This work includes addressing the mechanisms that control intracellular Ca2+ clearance (pumps and exchangers) through the plasma membrane and how these Ca2+-pathways are altered in channelopathies.
References
- Bomfim GHS, Mendez-Lopez I, Fernandez-Morales JC, Padin JF, Jurkiewicz A, Jurkiewicz NH, et al. Electrophysiological properties and augmented catecholamine release from chromaffin cells of WKY and SHR rats contributing to the hypertension development elicited by chronic EtOH consumption. Eur J Pharmacol. 2017;803:65-77.
- Méndez-López I, Bomfim GHS, Musial DC, Arranz-Tagarro JA, Velasco-Martín JP, Regadera J, et al. Altered mitochondrial function, capacitative calcium entry and contractions in the aorta of hypertensive rats. J Hypertens. 2017;35(8):1594-608.
- Garcia AG, Garcia-De-Diego AM, Gandia L, Borges R, Garcia-Sancho J. Calcium signaling and exocytosis in adrenal chromaffin cells. Physiol Rev. 2006;86(4):1093-131.
- Miranda-Ferreira R, de Pascual R, de Diego AM, Caricati-Neto A, Gandía L, Jurkiewicz A, et al. Single-vesicle catecholamine release has greater quantal content and faster kinetics in chromaffin cells from hypertensive, as compared with normotensive, rats. J Pharmacol Exp Ther. 2008;324(2):685-93.
- Bertelli AA. Wine, research and cardiovascular disease: instructions for use. Atherosclerosis. 2007;195(2):242-7.
- Breuss JM, Atanasov AG, Uhrin P. Resveratrol and Its Effects on the Vascular System. Int J Mol Sci. 2019;20(7).
- Fogacci F, Tocci G, Presta V, Fratter A, Borghi C, Cicero AFG. Effect of resveratrol on blood pressure: A systematic review and meta-analysis of randomized, controlled, clinical trials. Crit Rev Food Sci Nutr. 2019;59(10):1605-18.
- Kapetanovic IM, Muzzio M, Huang Z, Thompson TN, McCormick DL. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol. 2011;68(3):593-601.
- Bomfim GHS, Musial DC, Méndez-López I, Jurkiewicz A, Jurkiewicz NH, Padín JF, et al. Chronic resveratrol consumption prevents hypertension development altering electrophysiological currents and Ca(2+) signaling in chromaffin cells from SHR rats. Cell Signal. 2020;76:109811.

