By I. Losito, R. Abbattista, C. De Ceglie, A. Castellaneta, C.D. Calvano and T.R.I. Cataldi, Department of Chemistry, University of Bari “Aldo Moro”, Bari, Italy
With a worldwide yearly production of about 3 million metric tonnes, involving more than 40 different countries (1), olive oil has become an appreciated and commercially relevant food product well outside the borders of its Mediterranean cradle, where it is a pillar of everyday diet. This success is related to the increasing awareness of benefits that a regular consumption of olive oil may have for human health, in turn related to the presence of several bioactive compounds (2). Among the latter, peculiar compounds known as secoiridoids have emerged for their beneficial effects, especially for their antioxidant and anti-inflammatory properties (2) . During oil extraction, the main secoiridoids naturally contained in olive drupes, known as oleuropein and ligstroside, are subjected to a combination of enzymatic and chemical processes, ultimately leading to four major compounds: the aglycones of oleuropein and ligstroside, oleac(e)in and oleocanthal (Figure 1).
After almost three decades of research, it has been clarified that chemically unstable aglycones, formed after the detachment of the glucose molecule from oleuropein and ligstroside catalysed by b-glucosidase (a process starting during olive drupes crushing, with the release of the enzyme), can be transformed into several isomeric compounds. At the same time, oleac(e)in and oleocanthal, two further bioactive secoiridoids, are generated during oil production. They lack the methoxy-carbonyl moiety linked to carbon #4 (see Figure 1), since it is lost through the combination of an enzymatic (the hydrolysis of a methyl ester catalysed by methylesterase) and a chemical (the decarboxylation of the resulting carboxylic acid) process. As evidenced in Figure 1, the four secoiridoids include a phenolic moiety in their structure, corresponding to either 3-hydroxytyrosol or tyrosol, according to the number of OH groups (two or one, respectively). However, their intriguing molecular complexity arises mainly from the other portion of their structure, the one actually related to the class of secoiridoids.
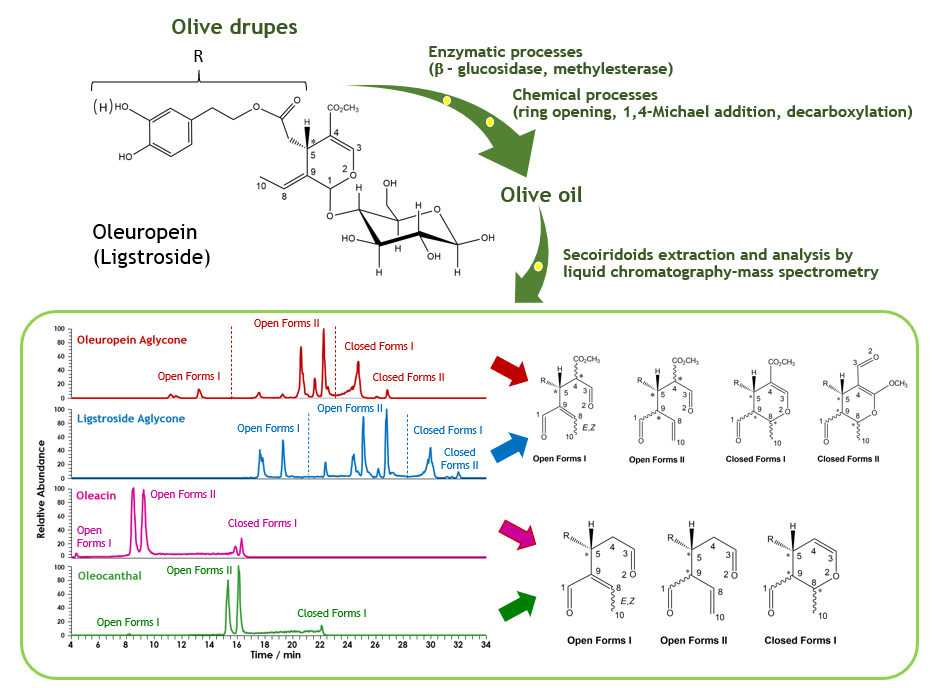
We have investigated this aspect in the last three years, as part of a research project on the valorization of Italian extra-virgin olive oils based on advanced analytical techniques (project VIOLIN) (3,4). Specifically, high resolution mass spectrometry coupled to liquid chromatography (LC-HRMS) has been adopted to separate and then identify isomeric forms corresponding to each of the four major secoiridoids of olive oil. As shown in Figure 1, and similarly to previous studies based on LC-(HR)MS, more than 10 different peaks were detected for compounds having the same molecular mass (isomers) arising from the aglycones of oleuropein and ligstroside, whereas 4-5 were found for oleacin and oleocanthal. The careful combination of previous literature information with data obtained by fragmenting the molecular structures of olive oil secoiridoids and analysing the resulting fragments (tandem mass spectrometry) helped in clarify the grouping of isoforms according to structural features (3,4).
Specifically, two types of open-structure forms were distinguished, differing just for the position of a C=C bond, which, in turn, is able to determine a different number of isoforms. This is due to the presence of an additional chiral centre (carbon #9) specifically on Open Forms II, that may couple with the chiral centres corresponding to carbon atoms #5 (the one originally included in the oleuropein and ligstroside molecules) and #4, thus leading to isomeric forms corresponding to diastereoisomers. The number of possible compounds is increased by the potential presence of both geometries (cis/trans) for the C8=C9 bond in the case of Open Forms I. On the other hand, two types of closed-structure (cyclic) forms were also identified, both including a dihydro-pyranic ring, including one or two C=O groups (type I and II, respectively). As for oleac(e)in and oleocanthal, the lack of a chiral centre on carbon #4, due to the loss of the methoxy-carbonyl group, reduced the number of possible isoforms, thus simplifying the chromatographic profiles of the two compounds (Figure 1). Interestingly, chromatographic profiles like those reported in Figure 1 were observed systematically for secoiridoids when extracts obtained from several Italian olive oils were analysed, although with some variations in the distributions of isoforms. MS responses were calculated for each compound by integrating all peaks detected in those profiles and then used to draw further interesting information, after a normalization based on the MS response of oleuropein, added as internal standard.
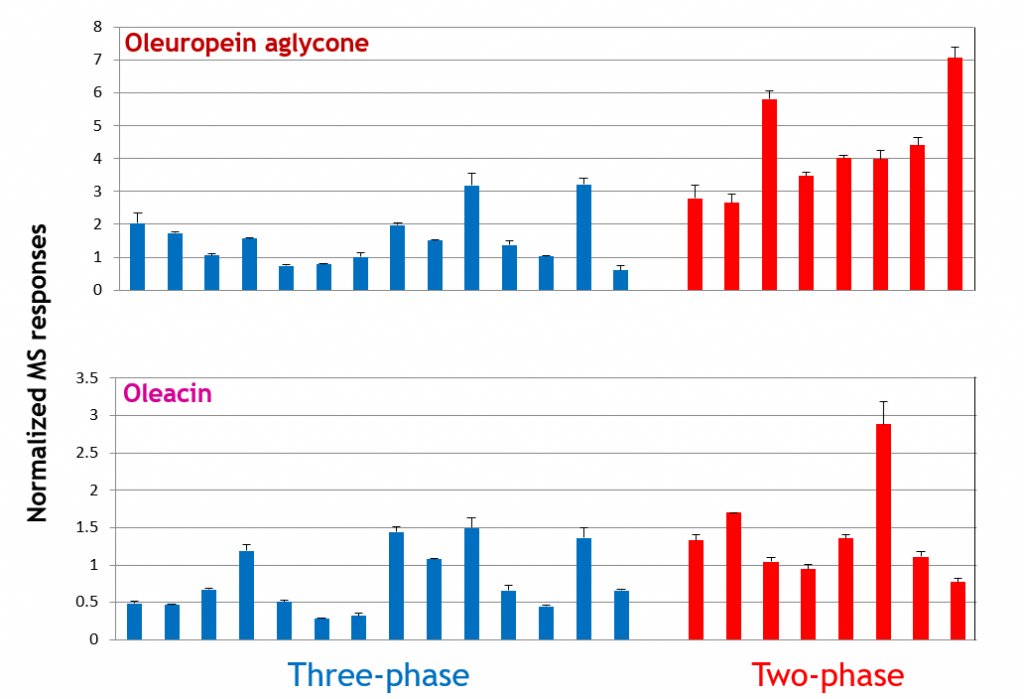
As an example, the effect on secoiridoids of horizontal centrifugation, a common approach to the separation of oil from olive paste, could thus be studied. Olive oils produced in Apulia, the region in Southern Italy leading the Italian olive oil production were considered. As shown in Figure 2, horizontal centrifugation based on the so-called two-phase decanters, that do not require the preliminary addition of water to olive paste, resulted in a general increase in the amount of oleuropein (and, similarly, of ligstroside) aglycones, compared to that performed using three-phase decanters, in which water is added in variable amounts. The effect was less evident for oleacin (see Figure 2) and oleocanthal (5).
Further interesting information was inferred when secoiridoids were analysed in olive oils produced in different Italian regions, using variable olive cultivars, and data obtained by LC-HRMS were processed using chemometrics, specifically Principal Component Analysis (PCA) (6).
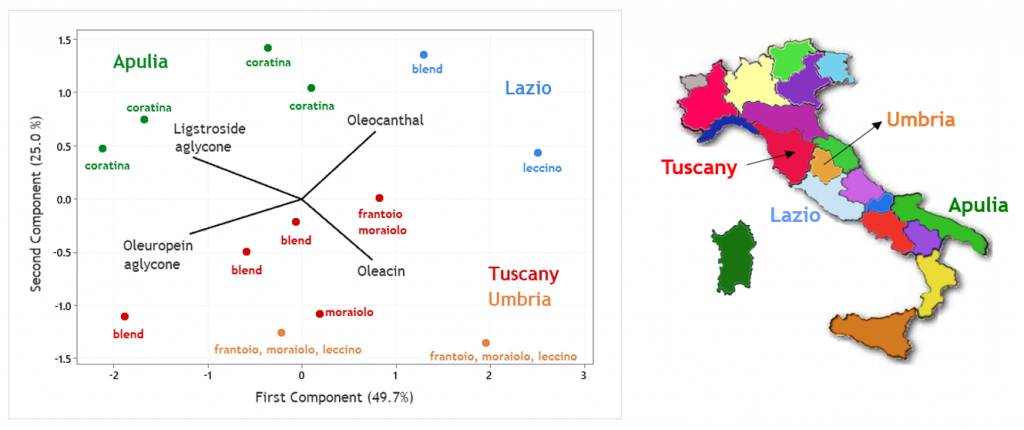
The location in the PCA biplot of olive oils produced, using two-phase decanters, in Apulia and in three regions of Central Italy, is shown as an example in Figure 3, with indication of cultivars adopted for each sample. As suggested by the contributions of secoiridoids MS responses to the first two principal components (reported as vectors in Figure 3), Apulian oils appeared generally richer in isoforms of ligstroside and oleuropein aglycones, due to the employment of drupes of Coratina cultivar, known to be naturally rich in those secoiridoids. Oleacin and oleocanthal were relatively more abundant in olive oils from Central Italy, produced with different cultivars (Leccino, Frantoio, Moraiolo), although some oils from Tuscany, produced using olive blends not disclosed by the respective producers, were found to contain relevant amounts of oleuropein aglycone.
The findings briefly described so far emphasize the wealth of information that can be obtained when such an intriguing class of compounds, like that of olive oil secoiridoids, is investigated by advanced analytical techniques like LC-HRMS. Since their bioactivity is known to be influenced by molecular structure, the ability to infer the profile of isoforms opens very interesting perspectives in terms of correlation between specific olive oils and beneficial effects for human health.
Funding: This research was funded by the Italian Ministero per l’Istruzione, l’Università e la Ricerca (MIUR), grant number PONa3_00395/1, and by Fondazioni in Rete per la Ricerca Agroalimentare (AGER), grant number AGER 2016-0169 (“VIOLIN” project).

Ilario Losito is Associate Professor in Analytical Chemistry and current Director of the SMART (Mass Spectrometry for Technology and Research) Inter-department Research Centre at the University of Bari “Aldo Moro”, Italy. He is also a member of the Board of the PhD School in “Soil and Food Science” of the same university. His main research interests currently focus on the characterization of peptides, proteins, lipids and other metabolites in food and biological matrices using Liquid Chromatography coupled to Electrospray Ionization High Resolution Mass Spectrometry (LC-ESI-HRMS). He is author of more than 100 papers in international scientific journals. In the last three years he has been involved in the VIOLIN research project, dealing with the valorization of Italian extra-virgin olive oil based on advanced analytical techniques. His research activity in the project is focused on extra-virgin olive oil bioactive compounds belonging to the class of secoiridoids.
References
- International Olive Oil Council statistical data, https://www.internationaloliveoil.org/wp-content/uploads/2020/12/HO-W901-23-11-2020-P.pdf
- Boskou, Olive and Olive Oil Bioactive Constituents, AOCS Press: Urbana, IL, 2015
- Abbattista et al., Talanta, 205 (2019) 120107
- Abbattista et al., J. Mass Spectr., 54 (2019) 843–855
- C. De Ceglie et al., J. Agr. Food Chem., 68 (2020) 3171-3183
- I. Losito et al., Molecules, 26 (2021) 743-763.


[…] See all at: https://www.ciencia-e-vinho.com/2021/03/31/secoiridoids-an-intriguing-class-of-bioactive-compounds-i… […]