By Lidia Daimiel Ruiz
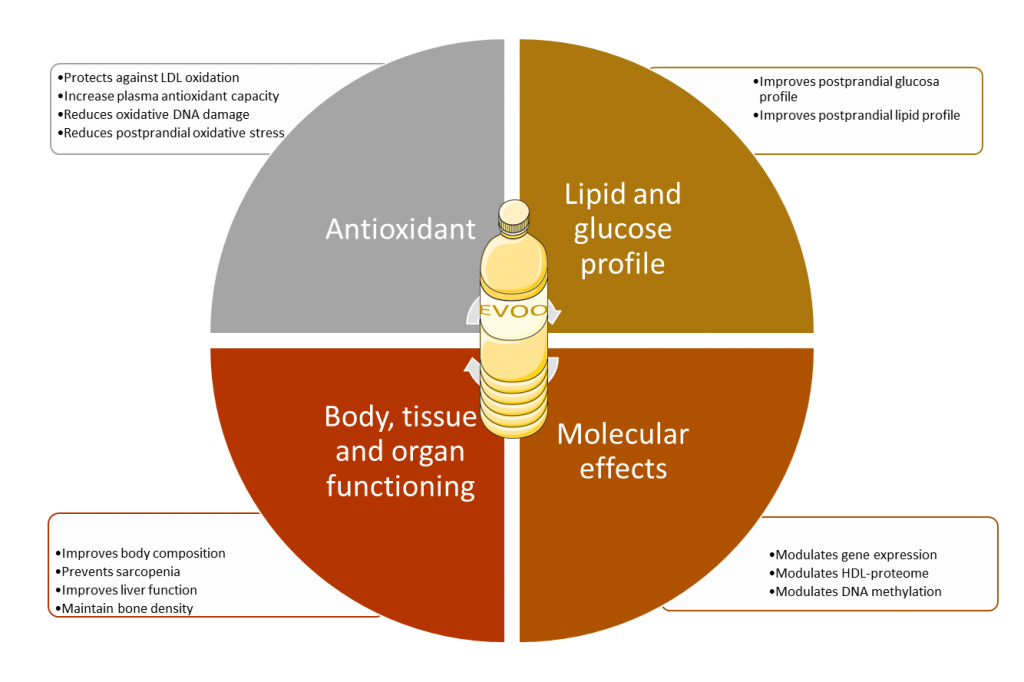
Extra-virgin olive oil (EVOO) has been named “the liquid gold” and is the basis of the Mediterranean diet. Currently, a plethora of evidence of the health properties of EVOO exist. In this regard, well-known studies as the Three-City and PREDIMED studies have shown that consumption of EVOO is associated with a lower incidence of cardiovascular disease (1,2). EVOO has antioxidant properties and, it has been shown to protect LDL against oxidation, to increase plasma antioxidant capacity, to reduce oxidative DNA damage and to increase anti-inflammatory HDL activity. EVOO has also been shown to improve postprandial oxidative stress, glucose, and lipid profile. EVOO has also been related to body composition, sarcopenia, liver function and bone density (3,4).
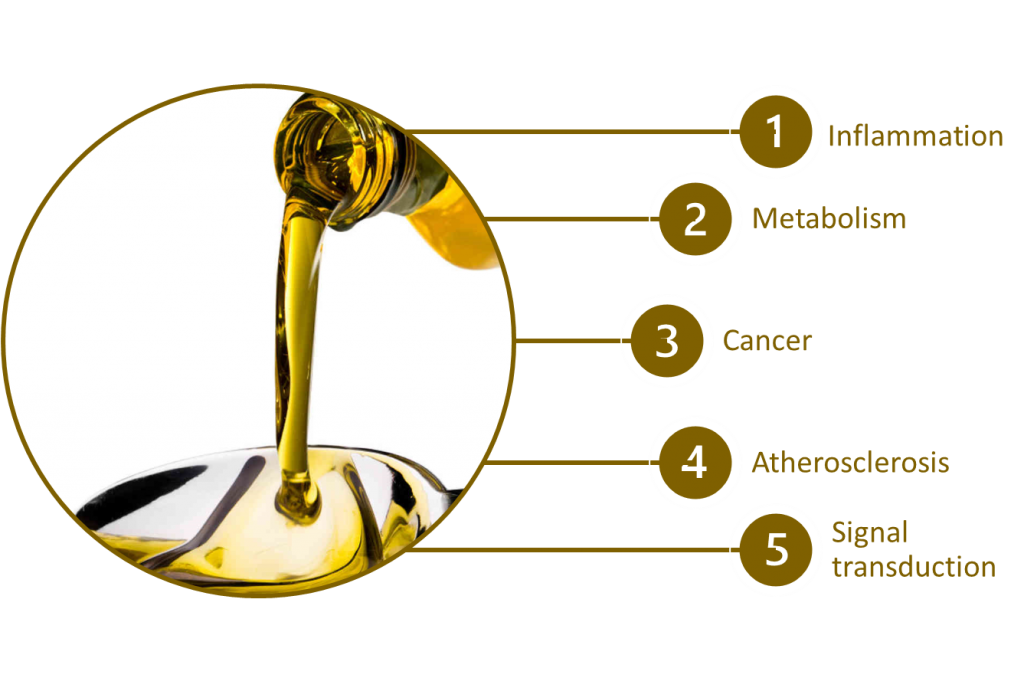
Although there are a plenty of intervention studies showing the benefits of EVOO consumption, little is known about the mechanisms that underlay such a health-promoting effect. The VOHF study (Virgin Olive Oil and HDL Functionality Study) has reported modulation of the expression of genes related to metabolism, inflammation, cancer, and atherosclerosis and of the HDL-proteome by extra-virgin olive oil (5–8). At epigenome level, PREDIMED study has shown that EVOO induces methylation changes in genes related to metabolism, signal transduction and inflammation (9). An acute ingestion of high-polyphenols EVOO intake modulates the transcription of blood cells genes involved in metabolism, inflammation, and cancer, suggesting a less inflammatory phenotype of blood cells (7,8).
The beneficial effects of EVOO have been attributed to its high content of polyphenols like hydroxytyrosol. Nevertheless, phenolic concentration in virgin olive oil is very variable and depends on different factors like cultivation area, irrigation techniques, the age of the olive tree, maturation of the olive fruit at harvest, the oil extraction process, storage conditions and cooking techniques.
We investigated how three differently polyphenol enriched EVOO modulated circulating microRNAs in the postprandial stage. Circulating microRNAs have a great potential as biomarkers of disease and nutritional status (10). microRNAs are short non-coding RNAs that regulate gene expression and are important fine-tuners of the metabolic state of the cell (11,12).
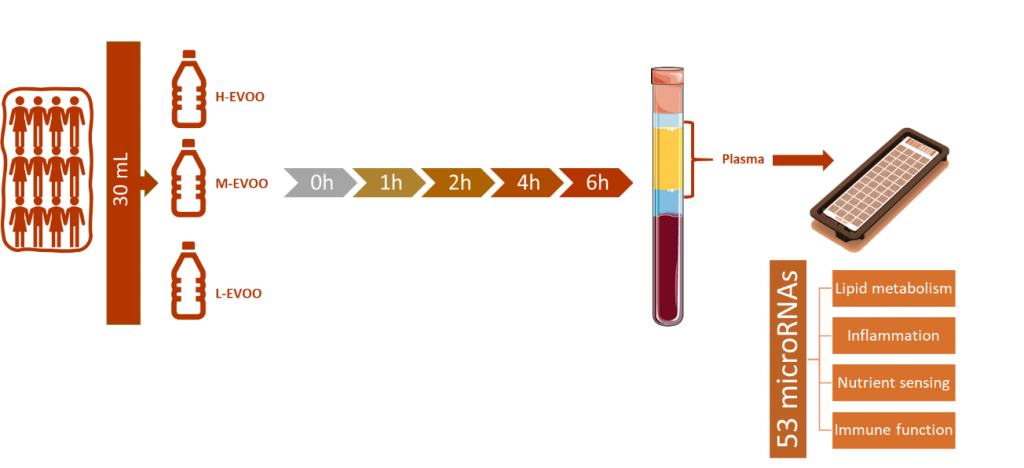
We measured a panel of 53 microRNAs related to cardiometabolic health and inflammation in plasma samples of the postprandial VOHF study. Samples were form 12 healthy volunteers (50% women, 22-60 years) that ingested of 30 mL of three differently enriched EVOOs after 12 hours of fasting. The three EVOOs differed in their content on polyphenols: high-EVOO (H-EVOO) contained 750 mg of total phenols/kg of oil, medium-EVOO (M-EVOO) contained 500 mg of total phenols/kg of oil and low-EVOO (L-EVOO) contained 250 mg total phenols/kg of oil. This study has some limitations, as the small sample size and the gender dimension and should be validated in larger cohorts including men and women. However, this study highlights the importance of microRNAs as mediators in the biological mechanisms modulated by nutrients and as potential biomarkers of nutrient ingestion.
We found some microRNAs consistently modified by the three functional olive oils, such as the cluster miR-17-92 or let-7e. The cluster miR-17-92 modulate glycolytic and oxidative metabolism (13). Let-7e is associated with metabolic syndrome and regulates endothelial function and immune activity (14,15). Interestingly, we found that changes in circulating microRNA levels were similar for L-EVOO and M-EVOO, while the highly enriched EVOO (H-EVOO) promoted different changes. We also found some correlation between circulating levels of microRNAs and HDL or oxLDL levels. In summary, our study suggests that microRNAs could be mediators of the biological effects of EVOO.
See all at https://doi.org/10.1002/mnfr.202000049

