By Paula Silva
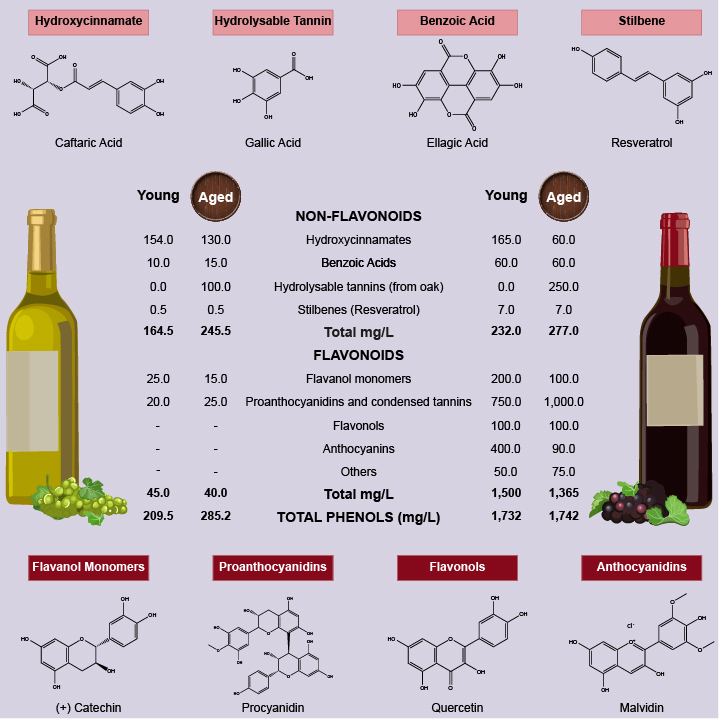
With aging decline of cognitive function occurs, but the mechanisms responsible are unknown. However, is now acknowledged that several lifestyle factors (e.g. diet, cognitive and physical activities) have an impact on brain aging and the development of neurodegenerative diseases. This post is about the neuroprotective abilities of the wine polyphenols (Figure 1) in correlation to the pathophysiological mechanisms involved in Alzheimer’s disease (AD) and summarizes a part of a recent review paper written by me in collaboration with David Vauzour published in Beverages Journal [1].
Alzheimer’s disease is the most common form of dementia and its prevalence increases with age. The pathological hallmark of AD is the extracellular deposition of amyloid-β aggregates which is the main component of senile plaques contributing to neuronal dysfunction and behavioural changes. Mechanisms that underlie the accumulation of amyloid-β in the brain are still not fully understood [2,3,4]. Looking for the proposed mechanisms involved in AD (Figure 2) several ones could be defined as potential targets for preventive/treatments strategies.
Major candidates’ therapeutic targets to lower amyloid-β in a preventive mode are secretases. Recent lines of research in the search for AD treatments, include β- and γ-secretase inhibitors and enhancers of α-secretase expression. A study with a mice model of AD shows that α-secretase activity is elevated in the brain of animals treated with Cabernet Sauvignon compared to ethanol-treated control [6]. α-Secretase is also elevated in primary neurons obtained from those mice [5]. Similar results are found in both in vitro and in vivo studies with resveratrol [7,8], quercetin [9], myricetin [10] and hydroxycinnamate compounds [11]. Several in vitro studies show that wine polyphenols are able of block β-Secretase (BACE 1) activity to decrease Aβ accumulation. However, few studies were carried out with animals to confirm that wine polyphenols could inhibit BACE 1. Analysing those in vivo studies, we can conclude that more experiments are needed to clarify whether BACE 1 can be a molecular target for wine polyphenols (1). In last year’s researchers are searching for γ-secretase inhibitors since this is a promising strategy for disease modification. Regarding wine polyphenols only in vitro studies with resveratrol were carried out and again different results are reported. Two studies show that resveratrol decrease γ-secretase processing activity [12, 13] and one study shows that resveratrol does not inhibit Aβ production, partly because it has no effect on the Aβ-producing enzymes β- and γ-secretases [14].
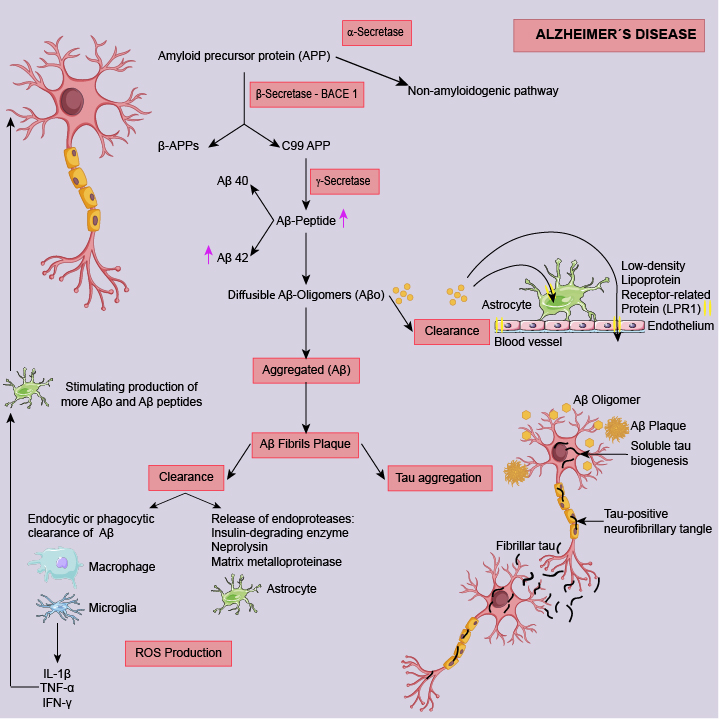
Aβ, amyloid beta; Aβo, amyloid beta oligomers; APP, amyloid precursor protein; IL-1β, interleukin 1
beta; IFN-γ, interferon gamma; LPR1, low-density lipoprotein receptor-related protein 1; ROS,
reactive oxygen species; TNF-α, tumor necrosis factor alpha.
Preventive and treatment options targeting amyloid aggregation and/or clearance are under study. Regarding wine polyphenols most of the studies used resveratrol that shows potential to both inhibition of Aβ oligomers aggregation and its clearance from the nervous system [1].
Tau protein is normally synthesized by neuronal cells to stabilize the microtubules for proper functioning of the neurons (Figure 2). So, targeting tau protein may prove to be a good therapeutic intervention. Neuroprotective effects of resveratrol also result from the inhibition of tau protein hyperphosphorylation, as it was observed cell nervous cell lines [15, 16]. Recently, some studies indicated the ability of quercetin [17], anthocyanins [18] and caffeic acid [19] to decrease tau protein hyperphosphorylation and thereby to attenuate the associated neuropathology.
Recent studies confirmed the influence of reactive oxygen species (ROS) on the development of AD. Targeting oxidative stress and increasing antioxidant defences of the brain would be an important strategy for maintaining survival of brain structure. Therefore, there has been an increase of interest in the therapeutic potential of polyphenols, especially resveratrol. As reviewed by me and David [1] It is known that resveratrol reduces ROS production in brain by:
-preventing nuclear factor-κB (NF-κB) activation;
-activating AMPK;
-reducing both lipid peroxidation levels and oxidative stress induced by Aβ senile plaques;
-downregulating inducible nitric oxide synthase (iNOS);
-inducing heme oxygenase 1 (HO-1);
-restoring intracellular antioxidant enzymes;
-reversing Aβ-induced malondialdehyde (MDA) overproduction derived from the lipid membrane oxidation.
Not only resveratrol but also the quercetin, (+)-catechin, epicatechin and myricetin are important in the prevention of neuronal damages by balancing oxidative and anti-oxidative status.
As suggested by epidemiological studies moderate consumption of wine, an element of the Mediterranean diet, is one of the main factors behind the neuroprotective effects observed in some populations [1]. Most of pre-clinical studies evaluated wine neuroprotection indirectly by analysing effects of the phenolic compounds of wine, mainly resveratrol. It is important to highlight that wine is a complex chemical mixture (Figure 1), therefore preventive/therapeutic efficacy of wine is due a combination of neuroprotective effects of wine phenolic compounds. In conclusion, wine could modulate multiple targets simultaneously involved in AD helping to prevent or slow age-related neurodegenerative diseases.
Those interested in a longer length report can download the working paper at:
https://www.mdpi.com/2306-5710/4/4/96

Paula Silva
Assistant Professor in the Laboratory of Histology and Embryology, Department of Microscopy, in the Institute of Biomedical Sciences Abel Salazar (ICBAS) of University of Porto (UPorto). Teaching experience covers: Histology and Embryology (Human and Comparative), Animal Models of Human Disease, and Science Communication. Director of the continuing training course “Science communication – Life and health sciences” (6ECTS) and of the continuing training unit “Animal Models of Human Disease” (6ECTS). She obtained her PhD in Biomedical Sciences in UPorto. Paula Silva presents in her CV 18 original articles published in journals indexed in the Science Citation Index (SCI), 1 book chapter, participation in some I&DT projects, and numerous works in many national and international congress. At present, her main research topic is the influence of moderate consumption of wine on chronic diseases, particularly, neurodegenerative diseases.
References:
- Silva, P.; Vauzour, D. Wine polyphenols and neurodegenerative diseases: an update on the molecular mechanisms underpinning their protective effects. Beverages 2018, 4(4), 96. https://doi.org/10.3390/beverages4040096
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO molecular medicine 2016, 8, 595-608, doi:10.15252/emmm.201606210.
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nature reviews. Immunology 2018, 18, 225-242, doi:10.1038/nri.2017.125.
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nature reviews. Neurology 2018, 10.1038/s41582-018-0013-z, doi:10.1038/s41582-018-0013-z.
- Wang, J.; Ho, L.; Zhao, Z.; Seror, I.; Humala, N.; Dickstein, D.L.; Thiyagarajan, M.; Percival, S.S.; Talcott, S.T.; Pasinetti, G.M. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2006, 20, 2313-2320, doi:10.1096/fj.06-6281com.
- Wang, J.; Ho, L.; Zhao, Z.; Seror, I.; Humala, N.; Dickstein, D.L.; Thiyagarajan, M.; Percival, S.S.; Talcott, S.T.; Pasinetti, G.M. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2006, 20, 2313-2320, doi:10.1096/fj.06-6281com.
- Porquet, D.; Casadesus, G.; Bayod, S.; Vicente, A.; Canudas, A.M.; Vilaplana, J.; Pelegri, C.; Sanfeliu, C.; Camins, A.; Pallas, M., et al. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. Age (Dordrecht, Netherlands) 2013, 35, 1851-1865, doi:10.1007/s11357-012-9489-4.
- Corpas, R.; Grinan-Ferre, C.; Rodriguez-Farre, E.; Pallas, M.; Sanfeliu, C. Resveratrol Induces Brain Resilience Against Alzheimer Neurodegeneration Through Proteostasis Enhancement. Molecular neurobiology 2018, 10.1007/s12035-018-1157-y, doi:10.1007/s12035-018-1157-y.
- Martin-Aragon, S.; Jimenez-Aliaga, K.L.; Benedi, J.; Bermejo-Bescos, P. Neurohormetic responses of quercetin and rutin in a cell line over-expressing the amyloid precursor protein (APPswe cells). Phytomedicine : international journal of phytotherapy and phytopharmacology 2016, 23, 1285-1294, doi:10.1016/j.phymed.2016.07.007.
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Multifunction of myricetin on A beta: neuroprotection via a conformational change of A beta and reduction of A beta via the interference of secretases. Journal of neuroscience research 2008, 86, 368-377, doi:10.1002/jnr.21476.
- Huang, Y.; Jin, M.; Pi, R.; Zhang, J.; Chen, M.; Ouyang, Y.; Liu, A.; Chao, X.; Liu, P.; Liu, J., et al. Protective effects of caffeic acid and caffeic acid phenethyl ester against acrolein-induced neurotoxicity in HT22 mouse hippocampal cells. Neuroscience letters 2013, 535, 146-151, doi:10.1016/j.neulet.2012.12.051.
- Choi, B.; Kim, S.; Jang, B.-G.; Kim, M.-J. Piceatannol, a natural analogue of resveratrol, effectively reduces beta-amyloid levels via activation of alpha-secretase and matrix metalloproteinase-9. Journal of Functional Foods 2016, 23, 124-134.
- Ohta, K.; Mizuno, A.; Ueda, M.; Li, S.; Suzuki, Y.; Hida, Y.; Hayakawa-Yano, Y.; Itoh, M.; Ohta, E.; Kobori, M., et al. Autophagy impairment stimulates PS1 expression and gamma-secretase activity. Autophagy 2010, 6, 345-352.
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. The Journal of biological chemistry 2005, 280, 37377-37382, doi:10.1074/jbc.M508246200.
- He, X.; Li, Z.; Rizak, J.D.; Wu, S.; Wang, Z.; He, R.; Su, M.; Qin, D.; Wang, J.; Hu, X. Resveratrol Attenuates Formaldehyde Induced Hyperphosphorylation of Tau Protein and Cytotoxicity in N2a Cells. Frontiers in neuroscience 2016, 10, 598, doi:10.3389/fnins.2016.00598.
- Jhang, K.A.; Park, J.S.; Kim, H.S.; Chong, Y.H. Resveratrol Ameliorates Tau Hyperphosphorylation at Ser396 Site and Oxidative Damage in Rat Hippocampal Slices Exposed to Vanadate: Implication of ERK1/2 and GSK-3beta Signaling Cascades. Journal of agricultural and food chemistry 2017, 65, 9626-9634, doi:10.1021/acs.jafc.7b03252.
- Shen, X.Y.; Luo, T.; Li, S.; Ting, O.Y.; He, F.; Xu, J.; Wang, H.Q. Quercetin inhibits okadaic acid-induced tau protein hyperphosphorylation through the Ca2+calpainp25CDK5 pathway in HT22 cells. International journal of molecular medicine 2018, 41, 1138-1146, doi:10.3892/ijmm.2017.3281.
- Ali, T.; Kim, M.J.; Rehman, S.U.; Ahmad, A.; Kim, M.O. Anthocyanin-Loaded PEG-Gold Nanoparticles Enhanced the Neuroprotection of Anthocyanins in an Abeta1-42 Mouse Model of Alzheimer’s Disease. Molecular neurobiology 2017, 54, 6490-6506, doi:10.1007/s12035-016-0136-4.
- Sul, D.; Kim, H.S.; Lee, D.; Joo, S.S.; Hwang, K.W.; Park, S.Y. Protective effect of caffeic acid against beta-amyloid-induced neurotoxicity by the inhibition of calcium influx and tau phosphorylation. Life sciences 2009, 84, 257-262, doi:10.1016/j.lfs.2008.12.001.