By Adeline Vignault, Jordi Gombau,Joan Miquel Canals, Pierre-Louis Teissedre and Fernando Zamora
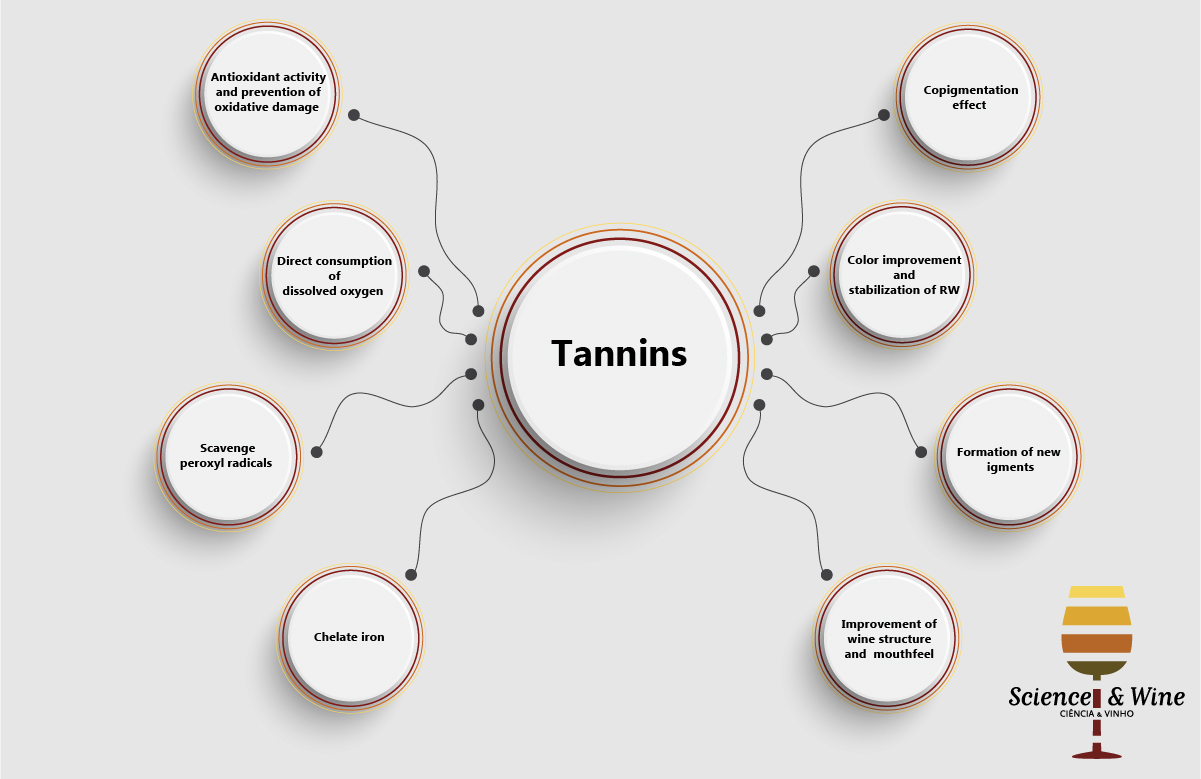
The use of oenological tannins in winemaking is a common practice but the International Organization of Vine and Wine (OIV) only authorize nowadays their use for wine fining [1]. Nevertheless, it is incontestable that oenological tannins are also currently used for many other purposes. Indeed, the literature has attributed several other functionalities to oenological tannins, such as antioxidant activity [2], direct consumption of dissolved oxygen [2,3], antioxidasic activity [4], ability to scavenge peroxyl radicals [5], ability to chelate iron (II), prevention of oxidative damage mediated by Fenton-based reactions [6], color improvement and stabilization of red wines [7], direct formation of new pigments [8], improvement of wine structure and mouthfeel [9], copigmentation effect [10], elimination of reduction odors [9] and even bacteriostatic effects [11].
Although oenological tannins are commonly employed to these ends, there are many questions about them that need to be clarified because many of these functionalities have only been verified empirically and there is a lack of scientific literature showing that oenological tannins really exert these functions. Moreover, under the term oenological tannin is included a wide range of phenolic compounds which differ in chemical structure, botanical origin, manufacturing process and probably in their effectiveness for the different described functionalities. This broad family of substances includes hydrolysable tannins (gallotannins and ellagitannins) from nut galls, tara, oak and chestnut, and condensed tannins (proanthocyanidins) from grape seeds and skins and other plant sources, such as quebracho, mimosa and acacia [8].
For that reason, the OIV working group on oenological tannins has performed an exhaustive study with 36 oenological commercial tannins to determine their chemical composition and verify their possible functionalities in order to include it in the OIV International Oenological Codex. Specifically this study has analyzed the functionalities of 17 proanthocyanidins comprising 9 procyanidins/prodelphinidins (PC/PD: 3 from grapes, 4 from grape seeds and 2 from grape skin) and 8 profisetinidins/prorobinetidins (PF/PR: 2 from acacia and 6 from quebracho), and 19 hydrolysable tannins comprising 8 gallotannins (GT: 4 from nut galls and 4 from tara) and 11 ellagitannins (ET: 8 from oak and 3 from chestnut). This post synthetize some of the main results obtained up to the current date. Specifically this post is focused in their chemical composition (Total Polyphenol Index [12], Bate-Smith [13], Methyl-cellulose [14], Folin-Ciocalteu [15], OIV official method [2] and phloroglucinolisis [16]), the antioxidant capacities (ABTS, CUPRAC, DPPH, FRAP and ORAC methods) [2], the direct oxygen consumption rate [2,3], the inhibitory effect on laccase activity [17] and the effectiveness as copigments to improve the color of red wines [10]. Some of these results have been previously published [2,3,10,18].
Figure 1 shows the principal component analysis corresponding to the chemical composition of the thirty-six different commercial tannins. This PCA enabled the different oenological tannins to be separated with only two incorrect classifications. More specifically, PC1 placed ET on the left and PC/PD on the right, with GT and PF/PR being in the center. PC2 enabled GT and PF/PR to be separated, locating GT above PF/PR.
Figure 1 shows the principal component analysis corresponding to the chemical composition of the thirty-six different commercial tannins. This PCA enabled the different oenological tannins to be separated with only two incorrect classifications. More specifically, PC1 placed ET on the left and PC/PD on the right, with GT and PF/PR being in the center. PC2 enabled GT and PF/PR to be separated, locating GT above PF/PR.
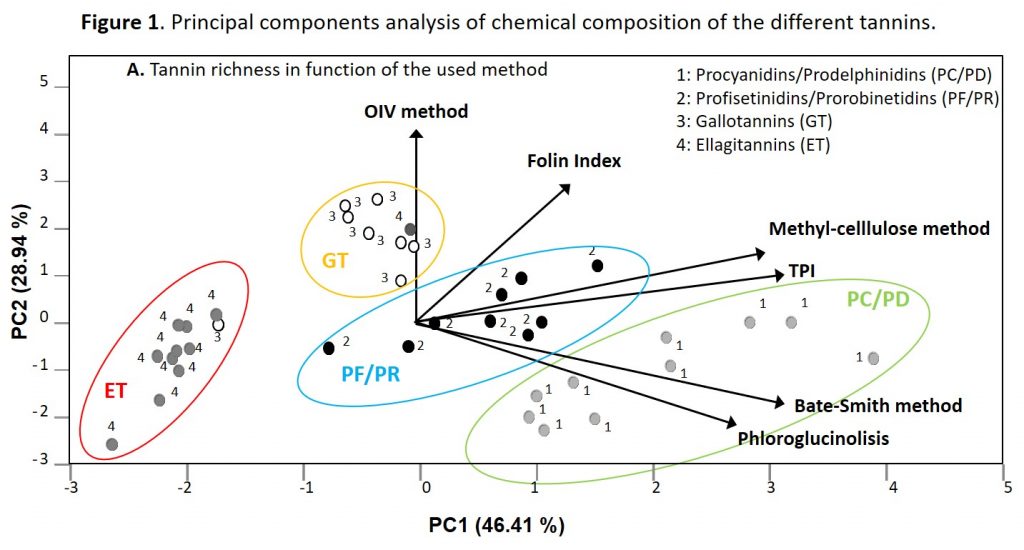
Figure 2 shows the principal component analysis corresponding to the antioxidant capacities of the 36 different commercial tannins. In that case, PCA enable to distinguish between hydrolysable and condensed tannins according to their antioxidant capacities, but cannot differentiate between PC/PD and PF/PR or between GT and ET. These data also indicates that hydrolysable tannins have higher antioxidant activity than condensed tannins.
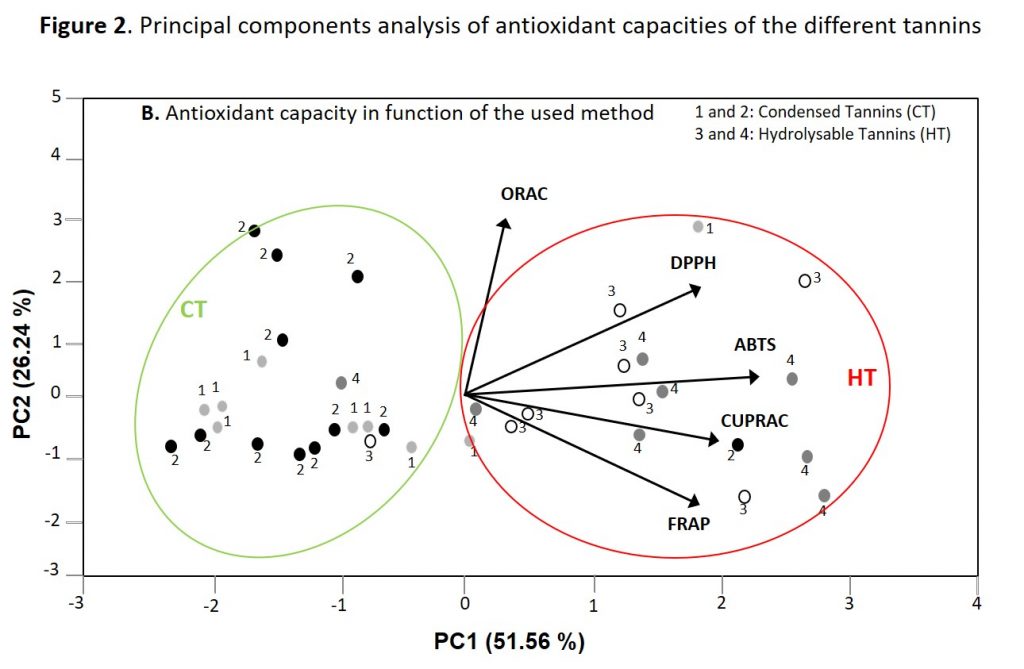
Figure 3 shows the oxygen consumption rate (OCR) of the different types of commercial tannins. This data confirms that ellagitannins are the most effective of the various oenological tannins, followed in decreasing order by condensed tannins (PC/PD and PF/PR) and finally gallotannins in terms of protecting the wine against chemical oxidation.
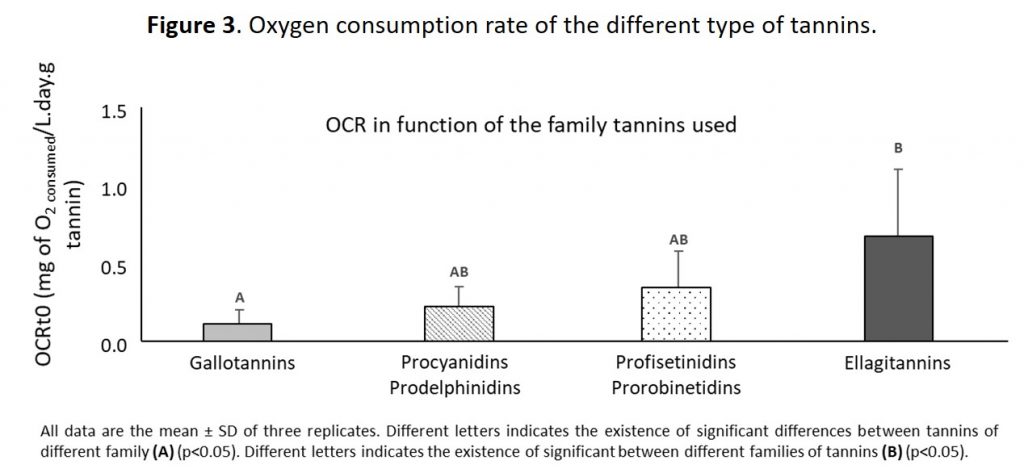
Figure 4 shows the inhibitory effect of the different types of commercial tannins on the laccase activity. All the tannins exerted an inhibitory effect on laccase activity which depended of the tannin dose. Indeed, the higher is the dose used lower is the laccase residual activity. This data confirms the utility of using oenological tannins to protect grape juice and wine from browning when grey root is present in the grapes.
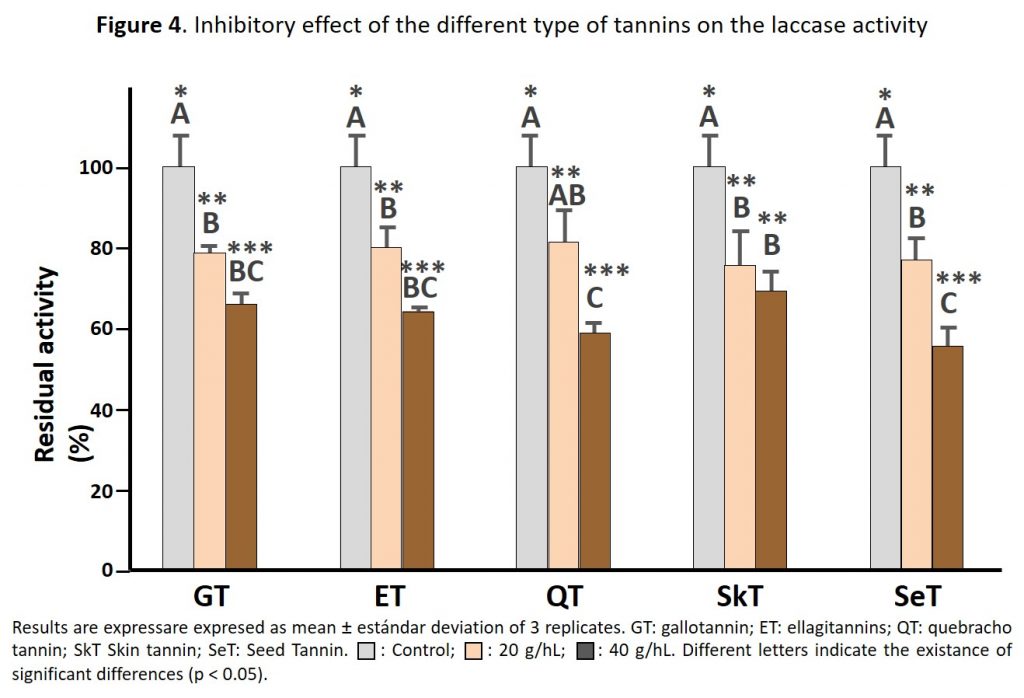
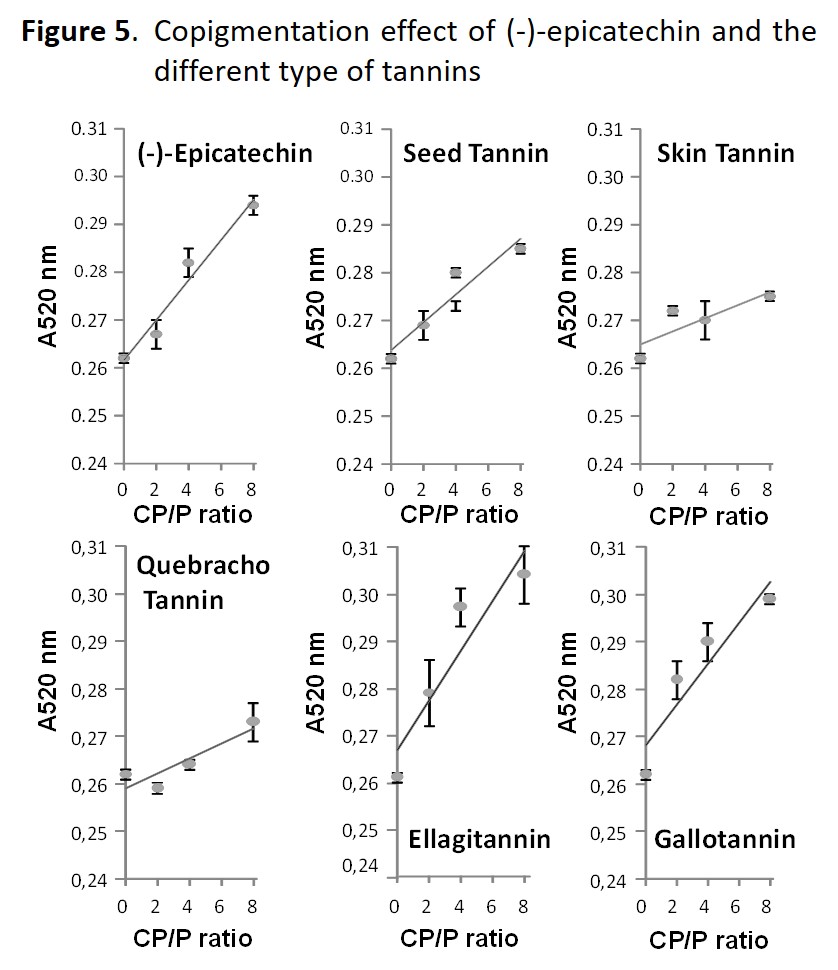
Figure 5 shows the effectiveness of the different type of commercial tannins as copigments in a wine model solution containing 50 mg/L of malvidin-3-O-glucoside. The results indicate that the presence of (-)-epicatechin and all the types of commercial tannins increase red color (A520nm) of the solution and that this increase is greater when the copigment/pigment ratio is higher. This data confirms therefore that all of them are good copigments and consequently it can be asserted that supplementation with commercial tannins exert a beneficial effect on the color of red wines.
It can be concluded that oenological tannins really exert a protection effect against wine oxidation because they have antioxidant activity, they consume directly oxygen and they exert an inhibitory effect on the laccase activity. Moreover, oenological tannins also exert a copigmentation effect which can improve and protect de color of red wines. Further studies are required to deep in the knowledge of the functionalities of oenological tannins but the actual findings suggest the need to include these new functionalities of oenological tannins in the OIV International Oenological Codex.

Adeline Vignault 1,2 
Jordi Gombau2 
Joan Miquel Canals 2 
Pierre-Louis Teissedre 1 
Fernando Zamora 2
1 Université de Bordeaux, Unité de recherche Œnologie, EA 4577, USC 1366 INRA, ISVV, 33882 Villenave d’Ornon cedex, France.
2 Departament de Bioquímica i Biotecnologia, Facultat d’Enologia de Tarragona, Universitat Rovira i Virgili, C/Marcel.li Domingo 1, 43007 Tarragona, Spain
Research Groups:
Adeline Vignault and Pierre-Louis Teissedre. Université de Bordeaux, Unité de recherche Œnologie
Jordi Gombau, Joan Miquel Canals and Fernando Zamora. Universidad Rovira i Virgili. Group of Research in Oenological Technology
Both research groups focus mainly their studies on the role of phenolic compounds on the color, astringency and general quality of red wines. Recently, they have begun a collaboration with the aim of studying the chemical characterization and functionalities of oenological tannins within the framework of the working group on oenological tannins of the International Organization of Vine and Wine (OIV).
References:
- International Oenological Codex. COEI-1-TANINS: 2015. http://www.oiv.int/public/medias/4093/e-coei-1-tanins.pdf.
- Vignault, M.R. González-Centeno, O. Pascual, J. Gombau, M. Jourdes, V. Moine, N. Iturmendi, J.M. Canals, F. Zamora, P.L. Teissedre, Food Chem. 268, 210-219 (2018)
- Pascual, A. Vignault, J. Gombau , M. Navarro, S. Gómez-Alonso, E. García-Romero, J.M. Canals, I. Hermosín-Gutíerrez, P.L. Teissedre, F. Zamora, Food Chemistry, 234, 26-32 (2017)
- Obradovic, M. Schulz, M. Oatey, Aust. N.Z. Grapegrow. Winemak. 493, 52–54 (2005)
- M. Magalhaes, I.I. Ramos, S. Reis, M.A. Segundo, Aust. J. Grape Wine Res. 20, 72–79 (2014)
- A. Perez, Y. Wei, M. Guo, J. Inorganic Biochem. 103, 326–332 (2009)
- Canuti, S. Puccioni, G. Giovani, M. Salmi, I. Rosi, M. Bertuccioli, Am. J. Enol. Vitic. 63, 220–231 (2012)
- Versari, W. du Toit, G.P. Parpinello, Aust. J. Grape Wine Res. 19, 1-10 (2013)
- Vivas, Rev. Fran. Oenol. 98, 11–14 (2001)
- Gombau, A. Vignault, O. Pascual, J.M. Canals, P.L. Teissedre, F. Zamora, F. BIO Web of Conferences 7, 02033 (2016)
- Lempereur, L. Blayteyron, B. Labarbe, C. Saucier, H. Klebek, Y. Glories, Rev Fran. Oenol. 196, 23–26 (2002)
- Ribéreau-Gayon, D. Dubourdieu, B. Donèche, (2006). Handbook of enology (2nd ed.). Chichester, West Sussex, England; Hoboken, NJ: John Wiley.
- Ribéreau-Gayon, E. Stonestreet, Chim. Anal. 48, 188–196 (1996)
- J. Sarneckis, R.G. Dambergs, P. Jones, M. Mercurio, M.J. Herderich, P. Smith, Aust. J. Grape Wine Res. 12, 39–49 (2006)
- Lorrain, G. Pasquier, M. Jourdes, L.G Dubrana, L. Gény, P. Rey, B. Donèche, P.L. Teissedre, Aust. J. Grape Wine Res. 18, 64–72 (2012)
- A. Kennedy, G.P. Jones, J. Agric. Food Chem. 49, 1740–1746 (2001)
- Urbano Cuadrado, P.M. Pérez-Juan, M. Luque de Castro, M.A. Gomez-Nieto, Anal. Chim. Acta 553, 99-104 (2005)
- Vignault1, O. Pascual, M. Jourdes, V. Moine, J.M. Canals, P.L. Teissedre, F. Zamora. Communication to the Congress GIENOL 2018. Ciudad Real, Spain (2018)