By Pauline Rousserie, Amélie Rabot and Laurence Geny-denis
Tannins, also called condensed tannins or proanthocyanidins (PAs) are polyphenolic compounds widely found in the plant kingdom. They are essentially secondary metabolites produced by plant for their adaptation and protection to biotic and abiotic stresses. In wine, they are among the most important quality factors due to their contribution to the organoleptic characteristics such as colour, astringency and bitterness1–3. Although tannins found in wine can come from microbial and oak sources, the main sources of polyphenols are grape skins and seeds4. Since the 1960s, this subject has been widely studied by a large number of researchers covering different types of wine, climate conditions, growing practices, and grape varieties. As these works have been conducted under different conditions, the data collected can be conflicting. This is why the question remains: what factors influence the biosynthesis, the quantity and the distribution of tannins in grape seeds and how can winemaking processes impact the extractability of seed tannins in wine?
Tannins result from the polymerisation of flavanols, also named flavan-3-ols which are the most reduced form of flavonoids. Tannins structure depend on the flavan-3-ols starter and extension units, the position and the stereochemistry of the linkage to the lower units, the degree of polymerization, and the presence of absence of modifications of the 3-hydroxyl group 5. The most commonly found monomeric units in PAs are (+)-catechin and (-)-epicatechin (table 1).
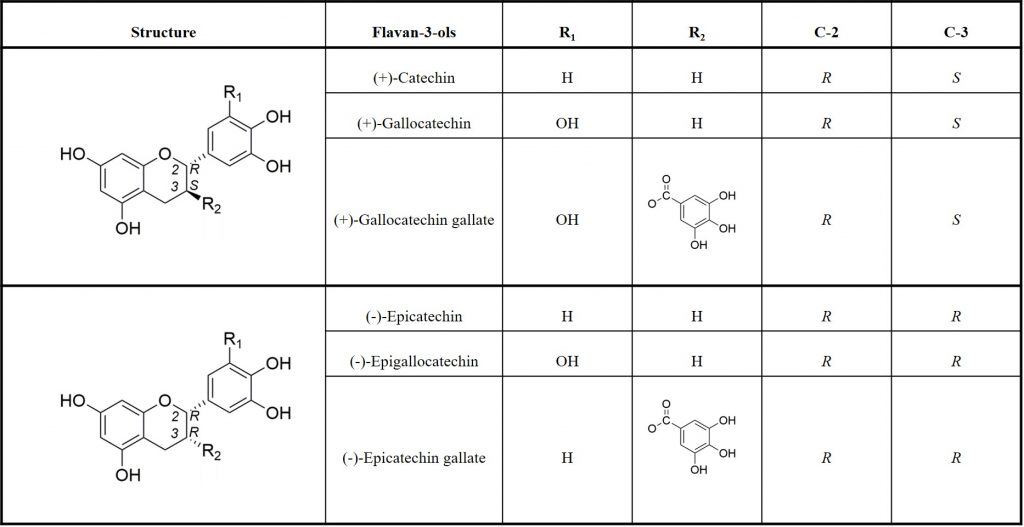
PAs are broadly distributed inside grapes and depending on the grape tissue location and the developmental stage of the berry, the quantity, the structure and the degree of polymerisation and galloylation of grape PAs differ. Indeed, at harvest time, the total of extractable phenolics in grape are distributed as follow: 10% or less in pulp, 28 to 35% in the skins and 60 to 70% in the seeds 6. Additionally, although differences are observed across varieties and vintages, it seems that at maturity, skin PAs present a significantly higher degree of polymerisation (from 2.1 to 85.7) than those of seeds (from 2.3 to 30.3). In terms of galloylation, differences between skin PAs and seed PAs at maturity have also been observed: seed PAs seem to present a higher percentage of galloylation (G%) (from 13.1 to 32.2%) than skin PAs (from 1.4 to 19%) 7.
Even though it is generally accepted that PA seeds are accumulated during the first berry growth phase, and decrease during the second growth phase, opinion on the evolution of the amount of seed tannins during ripening is divided. On one side, some authors report a significant decrease in the amount of PAs during the berry development, in some cases reaching 60% of the start amount. On the other side, a minority of authors have not found significant differences in PA seed content all along berry development. In cases where differences in polyphenol content are observed during berry maturation, observations show the same pattern consisting of two distinct periods, one of accumulation (from pea sized stage to veraison) and one of decline (from veraison to maturity) 8 (figure 1).
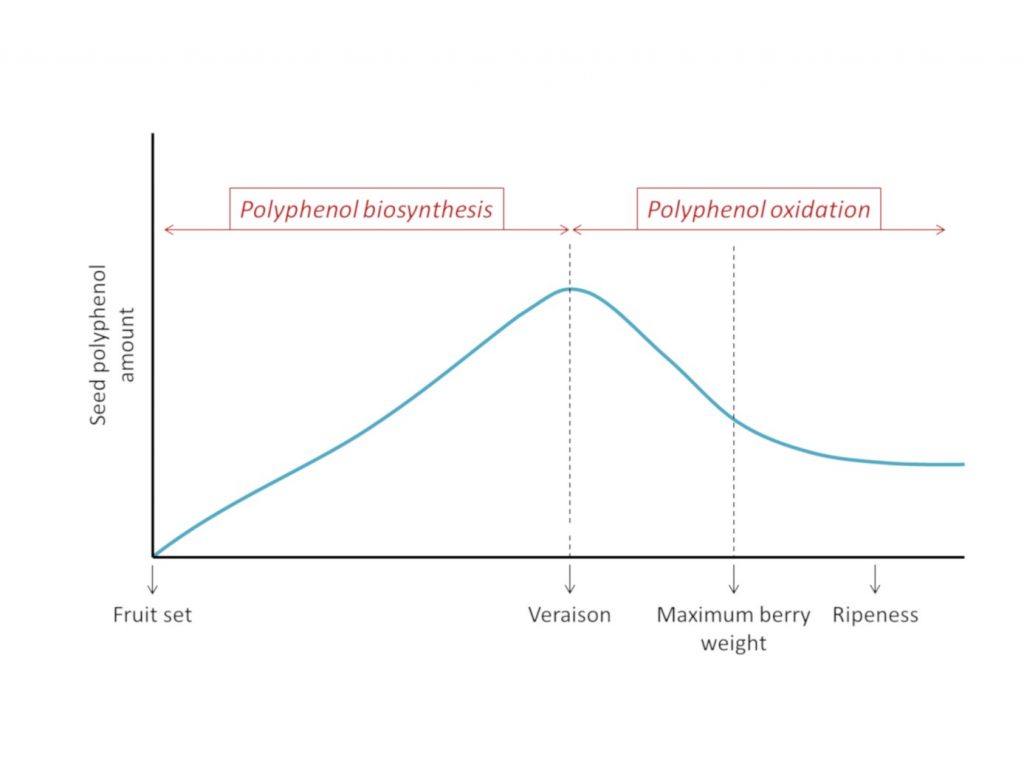
Furthermore, viticultural practices such as pruning, thinning, leaf removal and irrigation can impact the PAs seed biosynthesis and content7.
Because of the significant influence of tannins on red wine quality, many winemaking techniques have been developed as an alternative to the traditional elaboration of red wines. Some of this techniques such as fermentative maceration, cold maceration, enzyme addition, post fermentative maceration, high fermentation temperature, thermovinifcation, flash release and pulsed electric field are known improve the extraction of seed tannins (figure 2) 7.
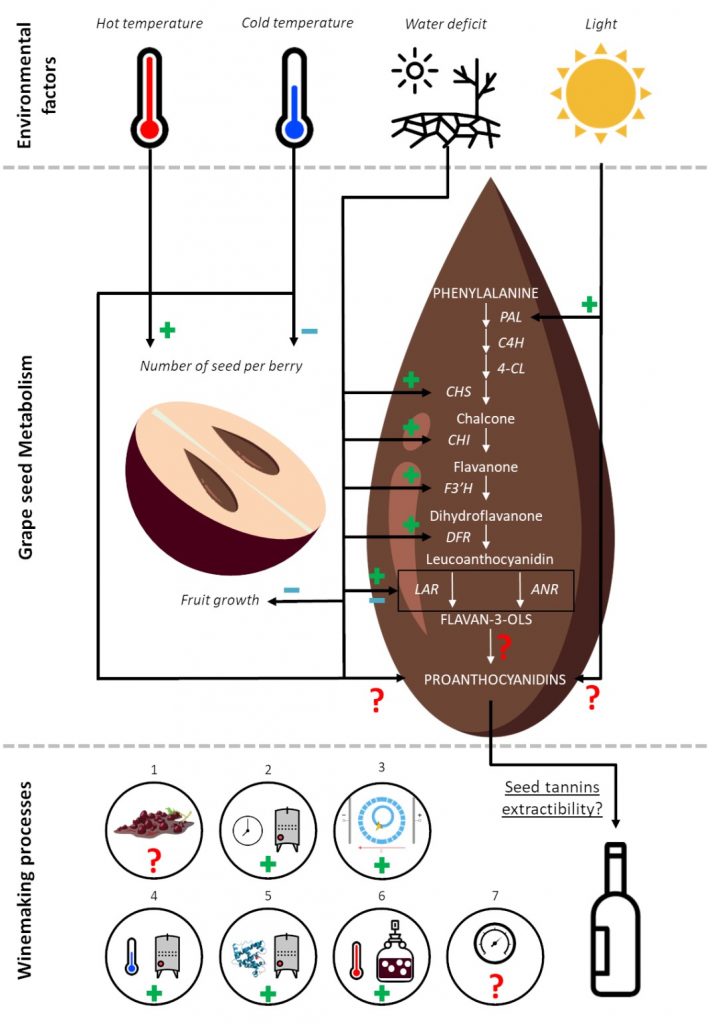
To conclude, due to a lack of knowledge, it remains difficult to estimate the seed phenolic maturity, even though the winemaker will take into account this maturity to determine the best harvest date. Indeed, no reliable, simple tool exists to determine the phenolic maturity of the seed, which is why winemakers often delay harvest time until the seeds turn uniformly brown. The consequence of this delay is an increase in the Brix level, leading to undesirably high ethanol levels during maceration. To get a better idea of the phenolic maturity of grape seeds and its impact on the sensory properties of wine, a robust study of the phenolic metabolism of seeds should be conducted. Knowledge of how tannins are metabolised by the grape seed will lead to the discovery of strong maturity markers which can be used to create a reliable tool to determine the phenolic maturity of seeds.
Those interested in a longer length report can download the working paper at:
https://pubs.acs.org/doi/abs/10.1021/acs.jafc.8b05768

Pauline Rousserie
PhD student working in oenology research unit of ISVV (Institut des Sciences de la Vigne et du Vin) at University of Bordeaux. Doctorate research project focused on the grape seed metabolism in various Vitis species to give no insight about tannins biosynthesis in seed, seed phenolic maturity and seed tannins extractability potential.

Amélie Rabot
Post graduate in plant physiology and PhD in Agronomy and Molecular Biology. Currently she is associate professor in Vine and Wine Sciences Institute in Bordeaux (France). Her main area of research is about the developmental process of grapeseeds. She focuses especially on biomolecules biosynthesis evolution during seeds ripening which could impact winemaking.

Laurence GENY-DENIS
Professor of grapevine physiology and viticulture at the Institut des Sciences de la Vigne et du Vin (ISVV) of Bordeaux University. Her research is focused on ripening process and impact of grape maturity on wine quality. She is co-authors of more 47 papers in international scientifics journals and had supervised 10 phD. She is coordinator of several research projects on grape quality in relation with technical practices as pruning and climate change. She supervises three diploma (a professional bachelor of winetourism, an international master of winetourism and a diploma of pruning (DUTE)) and coordinates the professional training at the ISVV.
References:
- Ma, W., Guo, A., Zhang, Y., Wang, H., Liu, Y. & Li, H. A review on astringency and bitterness perception of tannins in wine. Trends Food Sci. Technol. 40, 6–19 (2014).
- Soares, S., Brandão, E., Mateus, N. & de Freitas, V. Sensorial properties of red wine polyphenols: astringency and bitterness. Crit. Rev. Food Sci. Nutr. 57, 937–948 (2017).
- Teissedre, P.-L. & Jourdes, M. Tannins and anthocyanins of wine: phytochemistry and organoleptic properties. in Natural Products (eds. Ramawat, K. G. & Mérillon, J.-M.) 2255–2274 (Springer Berlin Heidelberg, 2013).
- Kennedy, J. A. Grape and wine phenolics: observations and recent findings. Cienc. E Investig. Agrar. 35, 107–120 (2008).
- Waterhouse, A. L. Wine Phenolics. Ann. N. Y. Acad. Sci. 957, 21–36 (2002).
- Shi, J., Yu, J., Pohorly, J. E. & Kakuda, Y. Polyphenolics in grape seeds—biochemistry and functionality. J. Med. Food 6, 291–299 (2003).
- Rousserie, P., Rabot, A. & Geny-Denis, L. From flavanols biosynthesis to wine tannins: what place for grape seeds? J. Agric. Food Chem. (2019).
- Kennedy, J. A., Troup, G. J., Pilbrow, J. R., Hutton, D. R., Hewitt, D., Hunter, C. R., Ristic, R., Iland, P. G. & Jones, G. P. Development of seed polyphenols in berries from Vitis vinifera L. cv. Shiraz. Aust. J. Grape Wine Res. 6, 244–254 (2000).
- Petrussa, E., Braidot, E., Zancani, M., Peresson, C., Bertolini, A., Patui, S. & Vianello, A. Plant flavonoids: biosynthesis, transport and involvement in stress responses. Int. J. Mol. Sci. 14, 14950–14973 (2013).
- Xia, E.-Q., Deng, G.-F., Guo, Y.-J. & Li, H.-B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 11, 622–646 (2010).
- Quideau, S., Deffieux, D., Douat-Casassus, C. & Pouységu, L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 50, 586–621 (2011).

