Introduction
Importance of Exploring Natural Compounds in Cancer Treatment
Cancer is a leading cause of mortality worldwide. A primary challenge in cancer therapeutics is that of the transformation of aggressive tumours into chronic illnesses [9]. Over the years, research in this field has evolved, and therapy currently extends beyond conventional treatments such as radiotherapy, chemotherapy, and surgical removal [10]. The escalating cancer rates highlight the crucial need to explore natural compounds for treatment that present themselves as an interesting option for prevention or in conjunction with pharmacological or conventional therapies [9,10]. Furthermore, the usage of natural products is a fundamental approach since they present accessibility and reduced cytotoxicity [10]. These compounds can originate from plants, animals, marine organisms, or microorganisms, and they target molecules found both in normal and tumour cells or molecules that only appear in cancer cell pathways [11,12].
Most drugs used in cancer treatment are only approved for adults as paediatric cancers are a limited area for investment [12]. However, several natural compounds have been the subject of research, specifically for NB [13]. For example, honokiol, obtained from several species of Magnolia, was found to inhibit the production of molecules associated with tumour progression and drug resistance in many types of cancers. In NB, it induces autophagic apoptosis through a tumour protein P53 (p53)-dependent pathway [14,15]. Another interesting example is that of products derived from garlic (Allium sativum), which include alliin that originates from cutting, crushing, or grounding garlic and reacts with the enzyme alliinase to form allicin. In NB cell lines, allicin reduced cell division and viability [16]. Moreover, foods from the MD present an interesting option for sources of natural compounds. For instance, in NB cells, an antioxidant that is present in red wine named resveratrol induced cell cycle arrest and apoptosis, and these effects were translated to mice, where the compound presented anticancer effects [17]. Another interesting example is that of lycopene, which is a pigment that is generally present in tomatoes and red fruits and has antioxidant properties, which are fundamental in protecting NB cells against oxidative damage and ER stress-induced damage [18]. In fact, the integration of these natural substances with established chemotherapy protocols offers a promising approach to enhance treatment efficacy and mitigate side effects, which are particularly relevant in paediatric cancer treatment [19].
Significance of Olive Oil in the Mediterranean Diet
In 2010, UNESCO recognised the Mediterranean Diet (MD) as an intangible cultural heritage of humanity [20]. The term MD was first introduced in 1960 by Ancel Keys, who considered it a diet with low levels of saturated lipids and the ability to diminish cholesterol levels in the blood, although the concept itself is thought to date back several millennia [21,22]. Years later, in 1995, Willet et al. described the MD as being a dietary regimen typical of certain regions of Italy and Greece with notably high life expectancy and low rates of diet-associated diseases [23]. Since the Mediterranean encompasses numerous regions, the cultures of different countries are seen in this diet, as well as a wide variety of ingredients with abundant antioxidants and anti-inflammatory nutrients. Fundamentally, the MD consists of an elevated consumption of vegetables, fruits, legumes, unrefined cereals, and nuts. The total fat intake must be lower than 30%, and the saturated fat intake must be even lower, not reaching 10%. The consumption of alcohol should be moderate, as should the intake of animal protein. Regarding energy content, recent descriptions account for around 2000 kcal/day, with 37% of the total calories accounted for corresponding to fat [21,22]. There are several factors that may have influenced the food elements present in this diet, which elucidate why it has been challenging to precisely define this dietary regimen [21]. General descriptions of the MD usually lack specific details such as proportions. However, certain habits, such as adding olive oil to vegetables and legumes to make them more pleasurable or eating fruit as dessert, were stated earlier by some authors. The description of the number of servings allowed for the design of a diet pyramid with different versions [24]. In 2011, the MD Foundation presented a new revised pyramid presentation, with the purpose of sharing a representation of the MD pyramid within the Mediterranean Region [20].
Olive oil is the main source of fat in the MD. In Ancient Greece, the olive tree was an essential symbol, and this component was used in medicine and religion, as well as in nutrition [23]. The composition of olive oil varies based on factors such as the origin of the olive tree or climatic conditions. In general, approximately 75% of its content consists of oleic acid (C18:1, n-9), a monounsaturated fatty acid (MUFA). It exhibits low percentages of saturated and polyunsaturated fatty acids [25]. In fact, the positive health effects associated with olive oil are linked to the consumption of extra virgin olive oil (EVOO), which contains high levels of the MUFA and 1–2% highly bioactive compounds. Regarding the MUFA, the existence of a single double bond allows for a higher resistance to oxidation and provides antioxidant properties. Bioactive compounds can be categorised into two groups based on their extraction process. The first consists of unsaponifiable compounds, such as squalene, sitosterols, and pigments, and corresponds to the fraction extracted with solvents after saponification of the oil. The second group consists of hydrophilic compounds, including tocopherol and phenolic compounds [26]. Tocopherol presents high vitamin E activity. Vitamin E is an antioxidant vitamin, with described preventive effects for cancer and cardiovascular diseases. Phenolic compounds, such as oleuropein (OLE), hydroxytyrosol (HT), and tyrosol (Tyr), may act as antioxidants in the human body because they possess a hydroxyl group with that function. These compounds are synthesised from tyrosine or phenylalanine and give a bitter and pungent taste to olive oil since they have a complex interaction with the taste buds [21,25,27]. Figure 1 presents examples of polyphenols that can be found in EVOO, as well as their chemical structure [28].
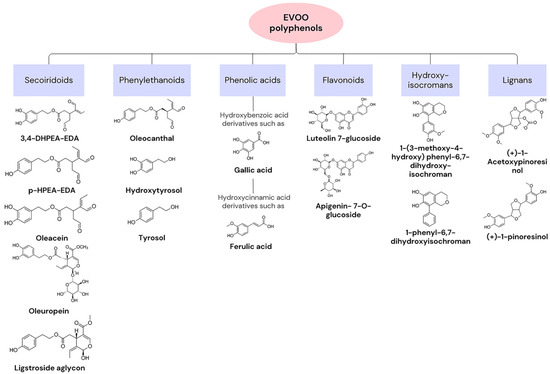
Figure 1. Categories of extra virgin olive oil (EVOO) polyphenols and examples with chemical structures.
Targeting aging processes, such as the reduction of telomers, changes in epigenetic patterns, cell senescence, and genomic instability, is one of the numerous abilities of olive oil in preventing disease [29]. These hallmarks of aging are often caused by reactive oxygen species (ROS), which, at elevated concentrations, cause damage to biomolecules such as DNA or proteins. The main production site of ROS is the mitochondria through the reduction of O2 to H2O during oxidative phosphorylation. As previously mentioned, the phenolic compounds in olive oil have antioxidant properties, allowing for the repression of damage caused by free radicals [21]. Moreover, olive oil has been associated with an increase in longevity and therapeutic health benefits for a variety of diseases. For example, in cardiovascular diseases, it lowers the plasma levels of the low-density lipoprotein (LDL) and increases the levels of the high-density lipoprotein (HLD), diminishing the risk of problems. This fundamental ingredient of the MD reduces the risk of cancer as well as inflammatory and autoimmune diseases [30]. The consumption of EVOO has been studied for its potential in the prevention or treatment of cancer. For instance, HT has demonstrated potent antiproliferative effects against human colon adenocarcinoma cells, suggesting a potential role in cancer treatment and prevention [31]. Moreover, the MD has been linked to a reduced incidence of breast cancer, highlighting its preventive potential [32]. Studies have also shown that EVOO phenols can suppress the migration and invasion of bladder cancer cells, providing insight into the potential of EVOO in cancer management [33]. Olive oil compounds have been found to inhibit vascular endothelial growth factor receptor-2 phosphorylation, which plays a crucial role in tumour angiogenesis [34]. Furthermore, phenols from EVOO have been shown to block cell cycle progression and influence the toxicity of chemotherapeutic drugs in bladder cancer cells [35]. The nutrigenomic approach suggests that different EVOO cultivars, owing to their varied phenolic content, could impact cancer prevention and treatment differently [36]. In summary, scientific evidence supports the potential of EVOO, particularly its phenolic compounds, in preventing and treating certain types of cancer. However, it is important to note that most of these studies focused on adult cancers and in vitro or in vivo models, so further research on paediatric cancers is needed.
Oleuropein and Hydroxytyrosol
Introduction to Oleuropein and Hydroxytyrosol
OLE, the primary phenolic compound found in olives (Oleaceae family), can constitute as much as 14% of their total weight [39]. It was discovered in 1908 by Bourquelot and Vintilesco and consists of three subunits as follows: secoiroid (elenolic acid), polyphenol (HT), and the glucose molecule. OLE formation in olives occurs as part of the secondary metabolism of terpenes. Within this metabolic pathway, the mevalonic acid cycle plays a crucial role, where a branching event gives rise to OLE [67]. In fact, the quantity of this compound in olives decreases with physiological development.
OLE metabolism can occur in the stomach or intestine depending on whether administration occurs through gastro-resistant capsules. When metabolism occurs in the stomach, either the acidic environment or the action of a β-glycosidase cleaves the β-glycosidic bond in OLE originating from the glucose and aglycone moieties. The aglycone transforms into two dialdehydes that are unstable and quickly converted into transposed secoiridoid, a lipophilic compound that under specific conditions such as prolonged exposure to an acidic environment can lose HT or methanol. If OLE is administered through capsules that resist the acidic environment of the stomach, it may reach the intestine unchanged. However, because OLE is a hydrophilic molecule, it cannot be absorbed by the cell membrane [39]. Although no clear evidence has shown that glucose transporters are specifically involved in the uptake of OLE, Hollman et al. (1995) stated that glucose transporters might be involved in the absorption of glycosylated biophenols [68]. Nevertheless, research has shown that in an isolated rat intestine, OLE is poorly absorbed [69].
As depicted in Figure 3, within the intestines, OLE undergoes transformation into HT and methyloleoside through lipase activity. Subsequently, another lipase converts the methyloleoside into oleoside and methanol.
OLE exhibits noteworthy versatility and pivotal roles in diverse biological processes. Some examples include its ability to decrease inflammation through the reduction in nuclear factor kappa-light-chain-enhancer (NF-kβ) activation and translocation to the nucleus, thereby resulting in the lack of expression of genes encoding inflammatory mediators. Additionally, it demonstrates neuroprotective activity since it increases the expression of NGF, BDNF, and their receptors TrkA and TrkB [70].
In cancer, OLE inhibits cell growth, motility, and invasiveness. OLE exerts its functions through, among other factors, the inhibition of NF-kβ and hypoxia-inducible factor 1α (HIF-1α), upregulation of pro-apoptotic genes and tumour suppressor miRNAs (miR-125b, miR-16, miR-34a, p53, p21, and TNFRS10B), and a decrease in the expression of histone deacetylases (HDAC) [71]. In prostate cancer, OLE is a pro-oxidant in neoplastic cells and an antioxidant in healthy cells. This results in decreased proliferation and cell death in tumours. Furthermore, OLE and HT contain an aromatic ring similar to that of oestradiol in their molecular structure, suggesting that these natural compounds may compete with oestradiol for binding to the oestrogen receptor (ER). This is particularly important in breast cancer, in which oestradiol stimulates cell growth. These polyphenols might inhibit cell proliferation in a concentration-dependent manner. Some studies have suggested that OLE induces cell death, especially in ER-negative tumours [39]. Figure 3 illustrates the further effects of OLE on distinct tumours.
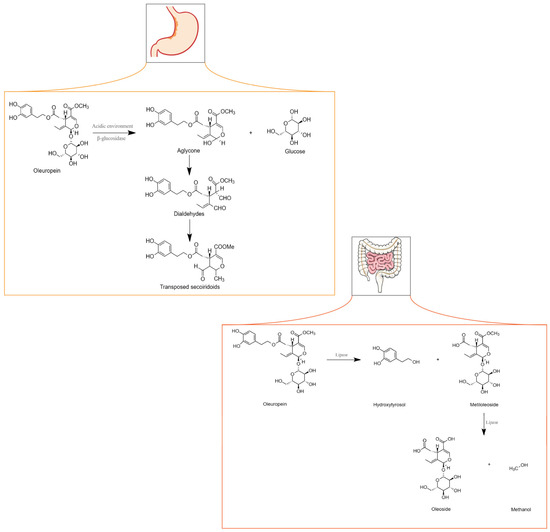
Figure 3. Metabolism of oleuropein in the stomach and intestine. Adapted from [71].
As previously mentioned, olive oil, under acidic conditions, originates from the polar phenolic compound HT. Nevertheless, HT may also result from the enzymatic breakdown of its respective glycosides. HT is an anti-inflammatory, antiatherogenic, and antithrombotic compound. Furthermore, it is slightly soluble in water as well as in lipids [72]. HT and OLE suppress LDL oxidation. However, while the concentration of the latter decreases with maturation of the olive fruit, the amount of HT increases [38].
Several methods have been developed for HT synthesis. One approach is to use lithium aluminium hydride to reduce the methyl ester of 3,4-hydroxyphenyl acetic acid. The absorption process occurs in the intestine, where the compound enters through bi-directional passive diffusion. HT can be oxidised to a 3,4-dihydroxyphenylacetaldehyde sulphate conjugate, 3,4-dihydroxyphenylacetic acid, glucuronide conjugate, homovanillyl alcohol, and homovanillic acid. Excretion predominantly occurs through the renal route, where the compound is eliminated in urine as glucuronide conjugates. At high concentrations, they may exhibit considerable toxicity [38].
In fact, previous research has demonstrated that HT can hinder the initiation and progression of tumours, which is in accordance with the described findings for OLE (Figure 4). It can prevent DNA from oxidative damage and induce apoptosis. Furthermore, the addition of sulphur-containing functional groups to HT may reverse resistance to anti-cancer drugs in leukaemia, suggesting that exploring potential beneficial structural modifications of HT might be an interesting approach in this field [73].
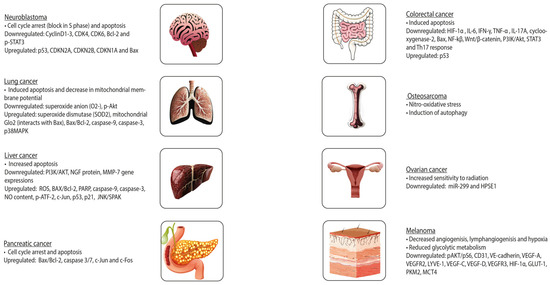
Figure 4. Effects of oleuropein on different tumours.
3.2. Sources and Bioavailability in Mediterranean Diet Foods
Both OLE and HT present low bioavailability [74,75]. They are present in table olives and olive oil. Notwithstanding, yeast produces HT during alcoholic fermentation by metabolising aromatic amino acids.
As previously mentioned, the decline in OLE and the rise in HT levels occurs as olives mature, establishing the latter as the predominant compound in mature olives. In fact, table olive processing results in the existence of three types of olives as follows: green olives, dark olives obtained through oxidation, or natural darkened olives. Green olives undergo lye treatment with a NaOH solution that leads to the conversion of OLE into HT and elenolic acid glucoside. Moreover, yeast and lactic acid bacteria that naturally exist in olives are also capable of metabolising OLE. The formation of olives darkened by oxidation implicates their preservation in brine (concentrated solution of sodium chloride in water) or an acidic solution and then darkening with air under alkaline conditions. The processing method surpasses the impact of both the variety and geographical origin on table olives’ phenolic compound content [76]. Generally, the mean content of OLE in black olives is 72 mg/100 g and 56 mg/100 g in green olives. Regarding HT, the average content is 659 mg/kg in black olives and 556 mg/kg in green olives.
The average OLE content in olive oil ranges from 0.17 mg/100 g in extra virgin oil to less than 1 µg/100 g in virgin varieties. Conversely, the average content of HT in olives is 3.5 mg/kg in virgin olive oils and 7.7 mg/kg in EVOOs. However, the amount of time in storage that precedes the analysis is an important factor to consider in determining the exact concentrations of OLE, HT, and other metabolites. Fresh EVOO presents higher concentrations of oleocanthal and oleacein and a lower quantity of HT and Tyr. Furthermore, cooking implies thermal oxidation that alters the phenolic compound content of olive oil, where HT derivates are among the initially depleted components [76].
Mechanisms of Antioxidant Action
The effectiveness of OLE and HT in providing antioxidant benefits is linked to their relative bioavailability. These phenolic compounds can act in two distinct ways, either by generating stable resonance structures through the scavenging of peroxyl radicals and the breaking of peroxide chain reactions or by averting the cooper sulphate-induced oxidation of LDL. The latter described mechanism consists of metal chelator activity, which is guaranteed since OLE and HT have hydroxyl groups capable of engaging in intramolecular hydrogen bonds with free radicals. The existence of these mechanisms was proved using metal-independent oxidative systems and stable free radicals [27,77]. Furthermore, HT induces the activity of detoxifying enzymes and promotes mitochondrial biogenesis, with both processes being fundamental in protecting against oxidative damage. HT can also regulate an adaptive signalling pathway that is activated in response to ER stress, and it also contributes to improving ER homeostasis [78]. The described antioxidant properties of these phenolic compounds have been proven to be stronger than those of vitamin E or butylated hydroxytoluene [27]. In cancer cells, at higher doses, OLE and HT have demonstrated a pro-oxidant activity, which is correlated with their anti-proliferative effects [78]. This pro-oxidant activity could also be associated with the cytotoxicity of the compounds at these high concentrations [79].
NRF2 can regulate protection against oxidation. In normal situations, Keap1 (Kelch-like ECH-associated protein 1) targets NRF2 and promotes its degradation in the proteasome. In stress-inducing situations, cysteine residues of Keap 1 are oxidized, thereby not allowing for its binding to NRF2 [80]. This results in the activation of NRF2, followed by translocation to the nucleus, where it binds to antioxidant response elements (AREs) and, subsequently, leads to the expression of antioxidant enzymes, such as SOD, c-glutamylcysteine synthetase (c-GCS), glutathione S-transferase (GST), and NADPH quinone oxidoreductase (NQO1). In in vivo studies, at a concentration of 5.0 mg/kg of HT, male mice C57BL/6J showed a decrease in oxidative stress since HT restored NRF2 and the levels of the peroxisome proliferator-activated receptor α (PPAR-α). When a higher concentration of HT was used, there was an increase in the activity of GST in the liver and muscle. In studies where OLE was utilized, there was an increased expression of enzymes regulated by NRF2 [77].
Read all at: Gonçalves, M.; Aiello, A.; Rodríguez-Pérez, M.; Accardi, G.; Burgos-Ramos, E.; Silva, P. Olive Oil Components as Novel Antioxidants in Neuroblastoma Treatment: Exploring the Therapeutic Potential of Oleuropein and Hydroxytyrosol. Nutrients 2024, 16, 818. https://doi.org/10.3390/nu16060818