By Diana Pimentel, Rute Amaro, Alexander Erban, Nuria Mauri, Flávio Soares, Cecília Rego, José M. Martínez-Zapater, Axel Mithöfer, Joachim Kopka, and Ana Margarida Fortes

Powdery mildew (PM) is one of the most dramatic diseases affecting grape production worldwide. PM is caused by the ascomycete fungus Erysiphe necator Schw. (syn. Uncinula necator [Schw.] Burr.), an obligate biotrophic fungus that infects berry clusters, and predisposes them to bunch rot infections (Gadoury 2012). Since most of the cultivars used for wine and table grape production belong to V. vinifera, PM is currently spread to all vinicultural regions, and the control strategy is entirely dependent on the widespread application of sulfur-based and synthetic fungicides resulting in environmental poisoning and health impacts.
During infection, the E. necator conidia form the appressorium that ruptures the cell wall, penetrates the plant cell, and then a feeding structure (haustorium) responsible for the dynamic exchanges between the fungus and the host cells is formed (Gadoury et al., 2012). Several defensive mechanisms to prevent pathogen penetration and colonization have been described in plants (Jones and Dangl, 2006). The two primary defense responses are the pathogen-associated molecular patterns (PAMP)-triggered immunity (PTI), and effector-triggered immunity (ETI). Both responses take place consecutively and are interconnected. However, most of the studies on responses to fungal infections are focused on grapevine leaves, and little is known about the defensive mechanisms in fruits (Agudelo-Romero et al., 2015).

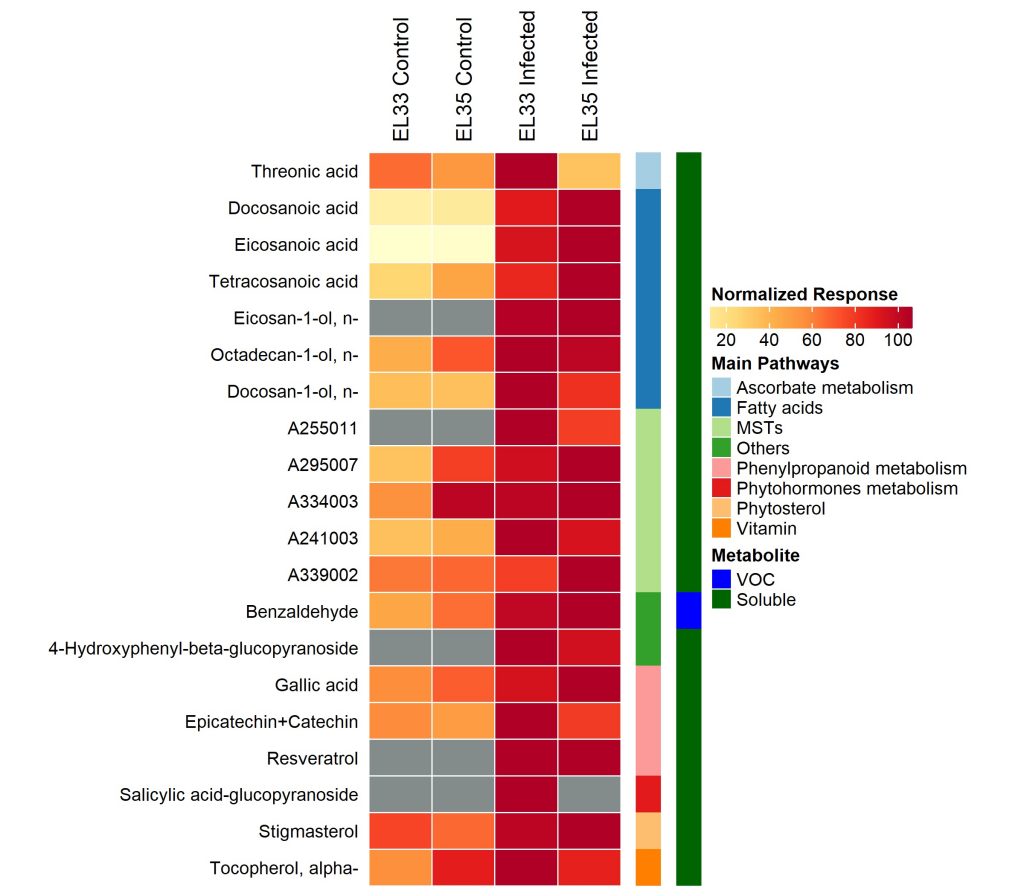
Mechanisms behind grapevine resistance or susceptibility are very complex, and despite the various studies performed so far, PM defensive responses remain unclear, particularly in infected fruits. Therefore, in this study, we applied high throughput technologies combined with targeted approaches to shed light on how extremely susceptible grape berries (V. vinifera cv. Carignan) respond to natural PM infection at the early stages of ripening. The transcriptional, hormonal, and metabolic data suggests the activation of defensive mechanisms in response to PM infection, as previously observed in leaves (Fung et al., 2008). However, certain responses namely fatty acid biosynthesis seems to be differently modulated in berries and leaves, suggesting organ-specific mechanisms. Furthermore, our results provided novel insights concerning the hormonal regulation of defense against E. necator with the putative involvement of jasmonates, often associated with response against necrotrophs (Coelho et al., 2019) in interaction with salicylic acid. On the other hand, the fungal metabolic program during infection involves secretion of effectors related to effector-triggered susceptibility, carbohydrate-active enzymes and activation of sugar, fatty acid and nitrogen uptake and could be under epigenetic regulation. This study also showed that E. necator altered specific ripening processes and identified potential metabolic biomarkers such as gallic, eicosanoic and docosanoic acids, and resveratrol, that can be used to monitor early stages of infection.
Grape and wine production are extremely important agronomic sectors in Portugal. Deeply integrated in the goals of our lab Fruit Functional Genomics and Biotechnology from Science Faculty of Lisbon University, we expect that this study provides valuable hints to develop grapevine plants that are more tolerant to powdery mildew by either strengthen defensive responses and/or reducing susceptibility. This information once validated may be used in modern breeding or subjected to gene editing. The final goal is to contribute to a more sustainable viticulture in a climate change scenario were diseases´ pressure is expected to increase.

Ana Margarida Fortes is currently an Assistant Professor at Department of Plant Biology of FCUL and leader of Fruit Functional Genomics & Biotechnology group (PFG, BIOISI). She has published 50 articles and five book chapters. H-index 22 (Google Scholar) and h-index 20 (Scopus). During her career she has developed research in several labs in Spain, Holland, Belgium, and Portugal. Her research interests have been focused on fruit ripening and defense. She has been studying tomato and grape ripening and grapevine defense responses when challenged with devastating fungal pathogens. The major goals of her research are the establishment of putative models of fruit ripening and fruit- pathogen interaction based on omics data to be further validated by functional analysis in particular gene editing. She got financial support as PI from 3 international (ERA-NET, H2020-MSCA-RISE, H2020PRIMA) and 3 national (FCT) projects on grapevine. Two international and one national are still undergoing and/or have been recently approved together with two COST Actions including the COST CA17111 – Data integration to maximise the power of omics for grapevine. She is currently the STSM coordinator of this COST Action. She made part of the Scientific Committee (SC) of several meetings, including the 10th Intl Symposium on Grapevine Physiology and Biotechnology (2016) and currently 11th Intl Symposium on the same topic. She was/ is the Co-PI and/or participant of several other national projects on grapevine. She has been reviewing manuscripts for major plant journals (Plant Cell, Plant Physiology, JXB) and science foundations (e.g. American NSF, German DGF, French ANR, and COST as expert/ rapporteur). She is since 2017 the Supervising editor of Plant Direct (Wiley, American Society of Plant Biologists and The Society for Experimental Botany). She has performed/ is performing editorial activities for other four Plant Journals. In 2016, her group received the first Innovation Prize from CNOIV for the work “Transcriptome and metabolome reprogrammig in Vitis vinifera cv. Trincadeira berries upon infection with Botrytis cinerea”.
References
Gadoury DM, Cadle-Davidson L, Wilcox WF, Dry IB, Seem RC, Milgroom MG. 2012. Grapevine powdery mildew (Erysiphe necator): a fascinating system for the study of the biology, ecology and epidemiology of an obligate biotroph. Molecular Plant Pathology 13, 1–16.
Jones JDG, Dangl JL. 2006. The plant immune system. Nature 444, 323–329.
Agudelo-Romero P, Erban A, Rego C, Carbonell-Bejerano P, Nascimento T, Sousa L, Martínez-Zapater JM, Kopka J, Fortes AM. 2015. Transcriptome and metabolome reprogramming in Vitis vinifera cv. Trincadeira berries upon infection with Botrytis cinerea. Journal of Experimental Botany 66, 1769–1785.
Fung RWM, Gonzalo M, Fekete C, Kovacs LG, He Y, Marsh E, McIntyre LM, Schachtman DP, Qiu W. 2008. Powdery Mildew Induces Defense-Oriented Reprogramming of the Transcriptome in a Susceptible but Not in a Resistant Grapevine. Plant Physiology 146, 236–249.
Coelho J, Almeida-Trapp M, Pimentel D, Soares F, Reis P, Rego C, Mithöfer A, Fortes AM. 2019. The study of hormonal metabolism of Trincadeira and Syrah cultivars indicates new roles of salicylic acid, jasmonates, ABA and IAA during grape ripening and upon infection with Botrytis cinerea. Plant Science 283, 266–277.