By Gaetano Leto
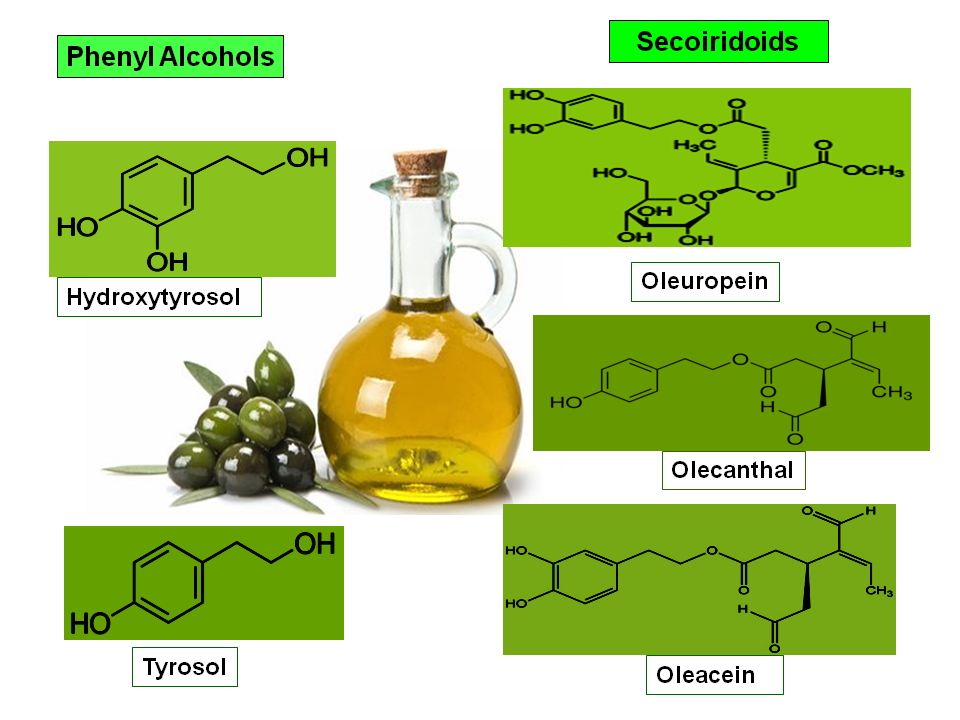
The skeleton represents the third most common target for bone metastasis after the lungs and liver [1]. Many types of solid tumours, in particular, breast, prostate, thyroid, lung, and kidney cancer preferentially metastasize to bone [1,2]. The incidence of bone metastasis by tumour type, reaches 65–90% in prostate cancer (PCa), about 65–75% in breast cancer (BCa), 60% in thyroid cancer, 30–40% in lung cancer, 40% in bladder, cancer and, 20–25% in renal cell carcinoma, while it is less frequent in other malignancies such as melanoma (11–17%) and colorectal tumours (10%) [2,3]. The median-survival time after diagnosis of bone metastasis from breast cancer, prostate cancer, thyroid cancer are 27 months, 25 months, and 23 months, respectively, while, in patients with renal, bladder, lung, and melanoma the median survival is lower (12 months, 8 months, 7-9 months and 6 months respectively) [2-4]. Furthermore, in some haematological malignancies such as multiple myeloma the rate of incidence reaches 70–95% while, the current 2-year survival rate is 87.1% [4-6]. Despite the therapeutic advances registered in recent years, the systemic treatments for cancer-related bone disease based on the administration of anti-resorptive drugs and/or anti-tumour agents and/or radiopharmaceutical, is still facing several drawbacks. In fact, these therapeutic options are merely palliative, and has been shown not to have a significant positive impact on patients’ survival [7]. In addition, these agents may negatively affect normal bone metabolism with detrimental consequences for cancer patients [8]. Hence, the need to discover new compounds which can effectively thwart tumour cell growth while, at the same time, overcoming drug-induced bone loss and preserving bone health [9,10]. In this setting, numerous preclinical studies have been undertaken with the aim of discovering new molecules based on natural products from the plant environment [11]. The advantages of using natural products for therapeutic purposes, in particular in cancer treatment, are manifold as these substances appear, in general, to be i) readily available, ii) non-toxic to normal human cells, iii)may act as multi-target agents, since they may affect different signalling pathways that control cancer progression [11-13]. In this scenario, a consistent number of clinical and observational studies have provided evidence on the beneficial effects on human health of several bioactive compounds present in olive oil (OO) [14,15]. Olives and OO are important components in the Mediterranean diet (MD) [16,17]. Consumption of OO has been associated with a reduced risk of developing non communicable diseases (NCDs) including cardiovascular, metabolic, neurodegenerative, bone diseases and cancer [14,15,17-19]. The beneficial effects of OO on human health, have been mostly attributed to the presence of numerous so-called “minor substances”, such as flavonoids, phenolic acids and alcohols, secoiridoids and lignans which are endowed with potent biological properties [14-19]. In particular, polyphenols such as Oleuropein, Hydroxytyrosol, and Oleocanthal have been shown to possess potent anti-inflammatory, antioxidant, antithrombotic, anti-microbial and anticancer properties [15,18-21] (Fig.1).
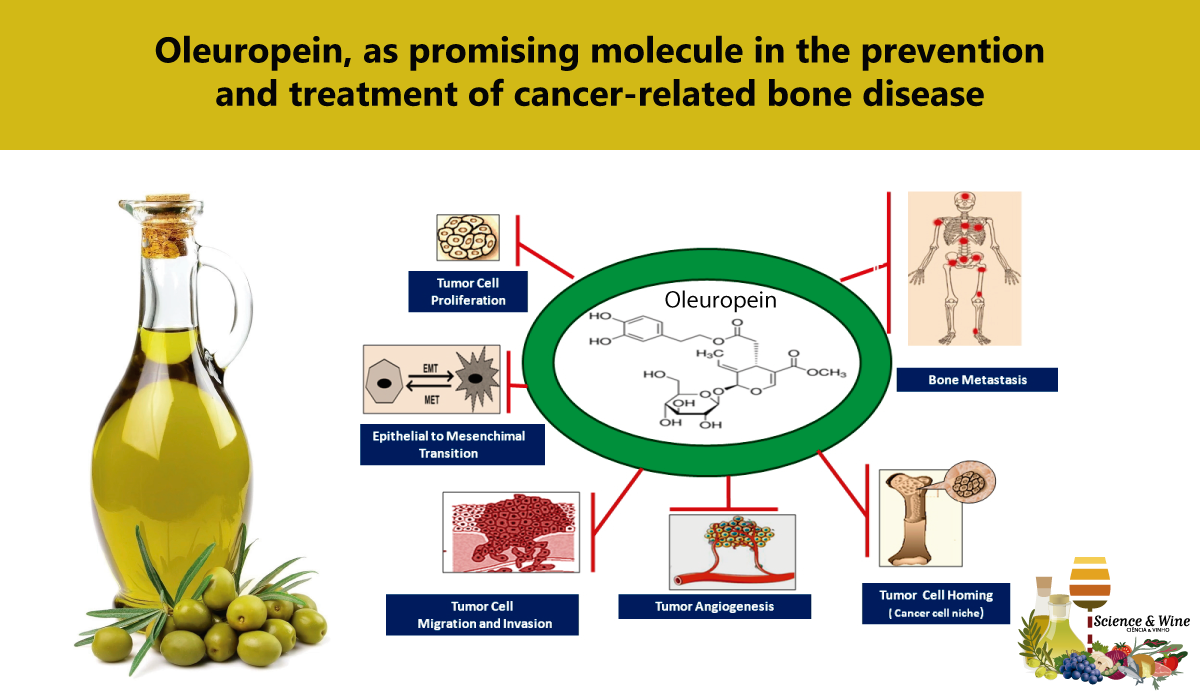
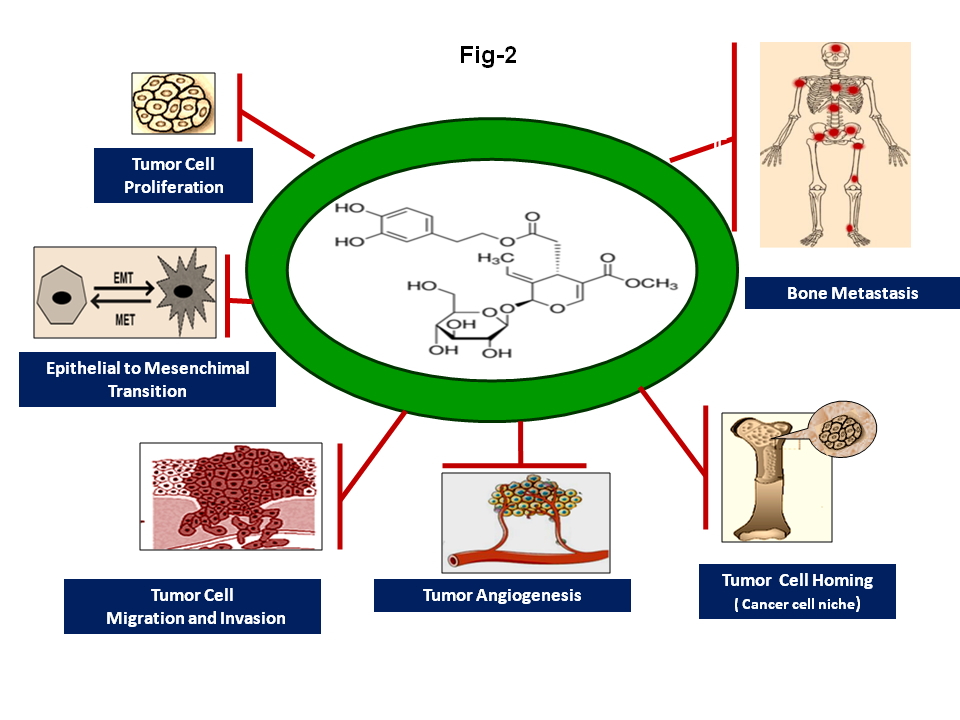
In this context, an increasing number of in vitro and in vivo studies have highlighted the fact that Oleuropein, the main bioactive phenolic compound present in olive leaves, the fruit and olive oil (and which is also responsible for bitter and pungent flavour of OO), can hinder the growth and spread of different types of human solid tumours [15,20-22]. Preclinical investigations carried out in order to elucidate the specific mechanisms underlying these effects, have shown that this molecule interferes with some key steps in the malignant progression, namely tumour cell proliferation, survival, angiogenesis, invasion and dissemination of cancer cells to distant organs, by down-regulating the expression and activity of various growth factors, transcription factors, hormones, cytokines, chemokines and enzymes fostering these processes [11-13,15,20-25] (Fig 2).
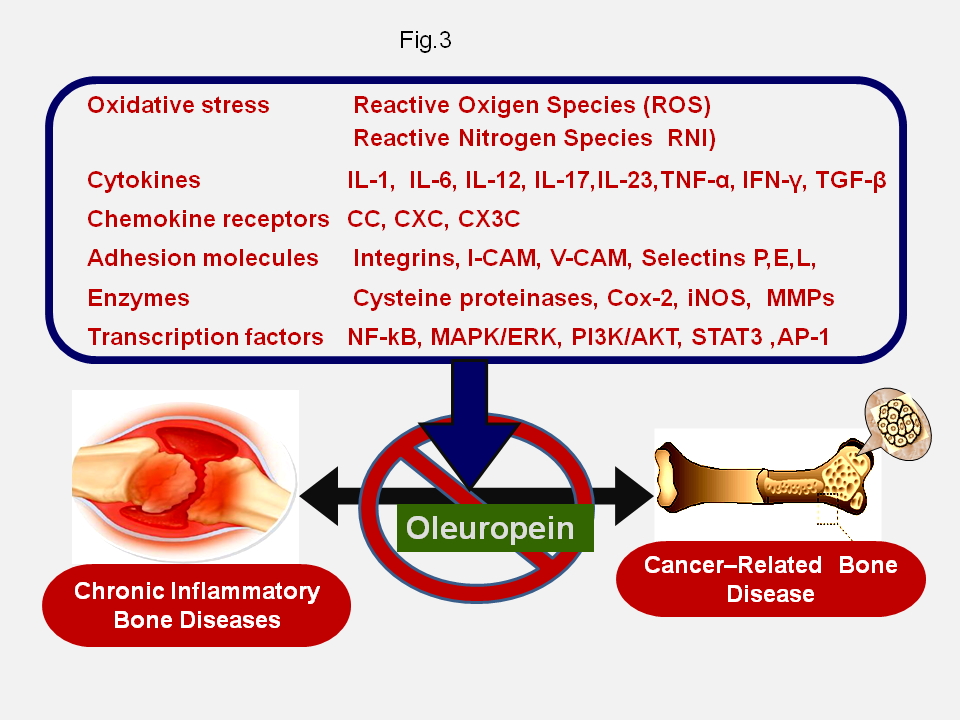
Interestingly, increasing experimental evidence has demonstrated that, most of these molecules appear to be involved in the formation of a permissive microenvironment, the so-called “(pre)metastatic niche”, which supports the homing, colonization and growth of disseminated cancer cells in the bone and, eventually, their subsequent survival, dormancy and resistance to clinical treatments [7,11-13,22,26] (Fig.2, Fig.3). Furthermore, recent reports have also highlighted the fact that these compounds appear to be involved in the regulation of bone turnover in normal conditions and in pathological processes associated with an excess of bone loss, such as inflammatory and autoimmune bone diseases, osteoporosis and cancer-related bone tumours [7,15,26,27] (Fig. 3).These findings are consistent with experimental and clinical observations showing that the protective effects of Oleuropein on bone health are probably due to the ability of this compound to modulate the expression and activity of these factors the expression of which proves to be deregulated in various pathological conditions associated with altered bone remodelling processes [11,15,26,28-31].
In this regard, recent in vitro studies have shown that Oleuropein can favour osteoblastogenesis by suppressing the expression of the peroxisome proliferator–activated receptor γ (PPARγ), which functions as a transcriptional regulator of adipocyte differentiation and osteoblast inhibitor, while inhibiting osteoclastogenesis [32,33]. Ultimately, these findings support the concept for a potential clinical usefulness of Oleuropein in the prevention and treatment of cancer-related bone disease. These data also suggest that the administration of Oleuropein in association with antitumor drugs and/or bone modifying agents and/or radiopharmaceuticals might result in improved therapeutic activity, in the prevention of bone loss induced by these agents and in a decreasing probability of developing drug resistance by malignant cells [8-10,21,23]. Although, the results from preclinical in vitro studies are promising in this regard, unfortunately, there is still a lack of in vivo studies, with the specific aim of evaluating the therapeutic effectiveness of Ole on metastatic bone disease by using suitable experimental animal tumour models. Nevertheless, these observations prompt the need for extensive future experimental and clinical investigations to assess the impact of Oleuropein on the clinical management and outcome of patients with skeletal metastases and further confirm the beneficial effects of the various components of the MD in preventing the onset of severe chronic disease.
See more on this subject in: Effects of oleuropein on tumor cell growth and bone remodelling: Potential clinical implications for the prevention and treatment of malignant bone diseases. Life Sci. 2020 Oct 30:118694. doi: 10.1016/j.lfs.2020.118694.

Prof. Gaetano Leto Education and Training
Correspondence: gaetano.leto@unipa
Education
- Degree cum laude in Biology, University of Palermo, Italy, (1977)
- Post graduate Scholarship in Pharmacology and Toxicology c/o Institute of Pharmacology, Faculty of Medicine, University of Palermo, Italy, (1981)
Professional Experience
- Laboratory Analysis c/o Navy Hospital, La Spezia, Italy (Military Service, 1978-79)
- Visiting Scientist, Department of Experimental Therapeutics, Roswell Park Cancer Institute, Buffalo, N.Y., USA (1985-87)
- Affiliate Researcher, Department of Experimental Therapeutics, Roswell Park Cancer Institute, Buffalo, N.Y., USA (1989)
- Senior Researcher, Faculty of Medicine University of Palermo (2002)
- Adjunct Professor of Pharmacology, Faculty of Medicine University of Palermo (2003)
Teaching Experience
- 1991/1996: Toxicology c/o School for Health Care Professional, University Hospital, Palermo, Italy
- 2003-2006; Cancer Chemotherapy c/o Faculty of Medicine, University of Palermo, Italy
- 2002 to present: Pharmacology and Chemotherapy c/o School of Medicine, University of Palermo, Italy
Professional membership
- American Association for Cancer Research (1996)
- Metastasis Research Society (1992)
- Italian Society of Pharmacology (2003)
Major research interest
- Biochemical mechanisms of tumour invasion and metastasis with emphasis on the role of cysteine and metallo- proteinases and growth factors in bone metastasis formation
- New potential circulating biomarkers of metastatic bone disease
- New antimetastatic agents (in particular, studies on chemo-preventive and anti-metastatic activity plant-derived polyphenols).
References
- R.E. Coleman. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 12( 2006) 6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931.
- J.F. Huang, J. Shen, X. Li et al. Incidence of patients with bone metastases at diagnosis of solid tumors in adults: a large population-based study. Ann. Transl. Med. 8 (7) (2020) 482. https://doi.org/10.21037/atm.2020.03.55.
- R.K. Hernandez, S.W. Wade, A. Reich et al. Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer. 18 (1) (2018):44. doi: 10.1186/s12885-017-3922-0.
- R.L. Siegel, K.D. Miller, A. Jemal. Cancer statistics. 2019. CA Cancer J. Clin. 69(1)(2019):7-34. doi:10.3322/caac.21551.
- SEER, Cancer Stat Facts: Bone and Joint Cancer. National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/statfacts/html/bones.html.
- R. Fonseca, S. Abouzaid, M. Bonafede et al. Trends in overall survival and costs of multiple myeloma. 2000–2014. Leukemia. 31 (9) (2017) 1915–1921. https://doi.org/10.1038/leu.2016.380.
- S. D’Oronzo, R. Coleman, J. Brown, F. Silvestris. Metastatic bone disease: Pathogenesis and therapeutic options. Up-date on bone metastasis management. J. Bone Oncol. 15(2019)4, https://doi.org/10.1016/j.jbo.2018.10.004. eCollection 2019 Apr.
- M.K. Skjødt, M. Frost, B. Abrahamsen. Side effects of drugs for osteoporosis and metastatic bone disease. Br. J. Clin. Pharmacol. 85 (2019) 1063–1071. https://doi.org/10.1111/bcp.13759.
- I. Makhoul, C.O. Montgomery, D. Gaddy, L. J. Suva. The best of both worlds – managing the cancer, saving the bone. Nat. Rev. Endocrinol. 12 (2016) 29–42. https://doi.org/10.1038/nrendo.2015.185.
- G. Leto. Current status and future directions in the treatment of bone metastases from breast cancer. Clin. Exp. Pharmacol. Physiol. 46 (2019) 968–971, https://doi.org/10.1111/1440-1681.13139
- P. Juárez, Plant-derived anticancer agents: a promising treatment for bone metastasis, Bonekey Rep. 3 (2014) 599. https://doi.org/10.1038/bonekey.2014.94.
- L. Sun, W. Zhou, H. Zhang et al. Modulation of Multiple Signaling Pathways of the Plant-Derived Natural Products in Cancer. Front. Oncol. 9(2019). 1153. https://doi.org/10.3389/fonc.2019.01153.
- A. Kapinova, P. Kubatka, A. Liskova et al. Controlling metastatic cancer: the role of phytochemicals in cell signaling, J. Cancer Res. Clin. Oncol. 145 (2019) 1087–1109. https://doi.org/10.1007/s00432-019-02892-5.h
- P. Reboredo-Rodríguez, A. Varela-López, T.Y. Forbes-Hernández et al. Phenolic Compounds Isolated from Olive Oil as Nutraceutical Tools for the Prevention and Management of Cancer and Cardiovascular Diseases. J. Funct. Foods 2018, 19, 2305
- M.L. Castejón, T. Montoya, C. Alarcón-de-la-Lastra, M. Sánchez-Hidalgo. Potential Protective Role Exerted by Secoiridoids from Olea europaea L. in Cancer, Cardiovascular, Neurodegenerative, Aging-Related, and Immunoinflammatory Disease. Antioxidants (Basel). 9 (2) (2020) 149. https://doi.org/10.3390/antiox9020149.
- C. Jimenez-Lopez, M. Carpena, C. Lourenco-Lopes et al. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014.
- A. Mazzocchi, L. Leone C Agostoni, I. Pali-Schöll. The Secrets of the Mediterranean Diet. Does [Only] Olive Oil Matter? Nutrients. 2019 Dec 3;11(12):2941. doi: 10.3390/nu11122941. PMID: 31817038; PMCID: PMC6949890.
- C. Nediani, J. Ruzzolini, A. Romani, L. Calorini. Oleuropein, a Bioactive Compound from Olea europaea L, as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants (Basel). 8(12) (2019) 578. doi:10.3390/antiox8120578.
- A. Maruca, R. Catalano, D. Bagetta et al. The Mediterranean Diet as source of bioactive compounds with multi-targeting anti-cancer profile. Eur. J. Med. Chem. 181 (2019) 111579, doi: 10.1016/j.ejmech.2019.111579. Epub 2019 Jul 3.
- I. Casaburi, F. Puoci, A. Chimento et al. Potential of olive oil phenols as chemopreventive and therapeutic agents against cancer: a review of in vitro studies. Mol. Nutr. Food Res. 57 (2013) 71–83. https://doi.org/10.1002/mnfr.201200503.
- H. Shamshoum, F. Vlavcheski, E. Tsiani, Anticancer effects of oleuropein, Biofactors. 43 (2017) 517–528. https://doi.org/10.1002/biof.1366.
- J. Torić, A.K. Marković, C.J. Brala, M. Barbarić. Anticancer effects of olive oil polyphenols and their combinations with anticancer drugs. Acta Pharm. 69 (2019) 461–482. https://doi.org/10.2478/acph-2019-0052.
- M. Imran, M. Nadeem, S.A. Gilani et al. Antitumor Perspectives of Oleuropein and Its Metabolite Hydroxytyrosol: Recent Updates. J. Food Sci. 83(2018) 1781–1791. https://doi.org/10.1111/1750-3841.14198.
- A. Ahmad Farooqi, S. Fayyaz, A.S. Silva et al. Oleuropein and Cancer Chemoprevention: The Link is Hot. Molecules. 22(2017), https://doi.org/10.3390/molecules22050705.
- R.A. Razali, Y. Lokanathan, M.D. Yazid et al. Modulation of Epithelial to Mesenchymal Transition Signaling Pathways by Olea Europaea and Its Active Compounds. Int. J. Mol. Sci. 20(14) (2019) 3492. https://doi.org/10.3390/ijms20143492.
- H. Roca, L.K. McCauley. Inflammation and skeletal metastasis. Bonekey Rep. 4 (2015) https://doi.org/10.1038/bonekey.2015.75.
- F. Coury, O. Peyruchaud and I. Machuca-Gayet. Osteoimmunology of Bone Loss in Inflammatory Rheumatic Diseases. Front. Immunol. 10(2019) 679. doi: 10.3389/fimmu.2019.00679.
- V. Nicolin, N. De Tommasi, S.L. Nori et al. Modulatory Effects of Plant Polyphenols on Bone Remodeling: A Prospective View From the Bench to Bedside. Front. Endocrinol. (Lausanne). 10 (2019) 494. https://doi.org/10.3389/fendo.2019.00494.
- K.Y. Chin, S. Ima-Nirwana. Olives and Bone: A Green Osteoporosis Prevention Option. Int. J. Environ. Res. Public Health. 13 (2016). https://doi.org/10.3390/ijerph13080755.
- K.Y. Chin, K.L. Pang. Therapeutic Effects of Olive and Its Derivatives on Osteoarthritis: From Bench to Bedside. Nutrients. 9 (2017). https://doi.org/10.3390/nu9101060.
- N. Yahfoufi, N. Alsadi, M. Jambi, C. Matar. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients. 10 (11) (2018) 1618. https://doi.org/10.3390/nu10111618.
- O. García-Martínez, E. De Luna-Bertos, J. Ramos-Torrecillas et al. Phenolic Compounds in Extra Virgin Olive Oil Stimulate Human Osteoblastic Cell Proliferation. PLoS One. 11 (2016) e0150045. https://doi.org/10.1371/journal.pone.0150045.
- R. Santiago-Mora, A. Casado-Díaz, M.D. De Castro, J.M. Quesada-Gómez. Oleuropein enhances osteoblastogenesis and inhibits adipogenesis: the effect on differentiation in stem cells derived from bone marrow. Osteoporosis Int. 22 (2011) 675–684. https://doi.org/10.1007/s00198-010-1270-x.