By Dr Stéphanie Weidmann-Desroche and Sandrine Rousseaux
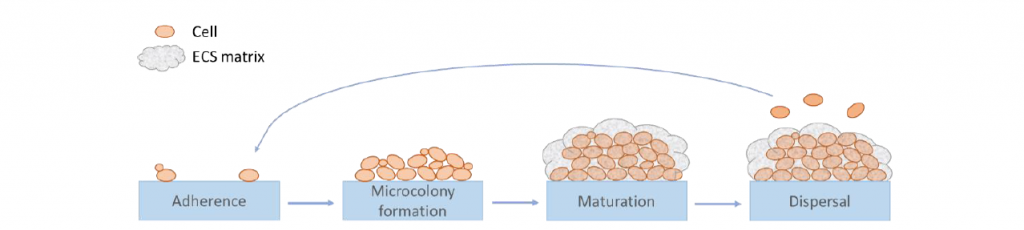
Biofilms are complex associations of single- and multiple- species interconnected cells embedded in a hydrated self-produced matrix established at solid/liquid or liquid/air interfaces. Biofilm development is a dynamic process including the key steps of (i) the adhesion (ii) the maturation of microcolonies in a three-dimensional structure, and (iii) the detachment during which cells acquire a particular phenotype (Figure 1). Extracellular substances (ECS) produced throughout biofilm development are mainly composed of polysaccharides, proteins, extracellular DNA and lipids. Biofilm mode of life allows microorganisms to better adapt to environmental conditions, especially chemical and physical resistance. This growth strategy, through the surface colonization and the increase of stress resistance, contributes to the persistence of microorganisms in different environments, such as those encountered in the food industry. Many studies have investigated the presence of biofilms, especially in the case of negative effects due to the risk of recurrent contamination of food and raw materials by pathogenic or spoilage species.
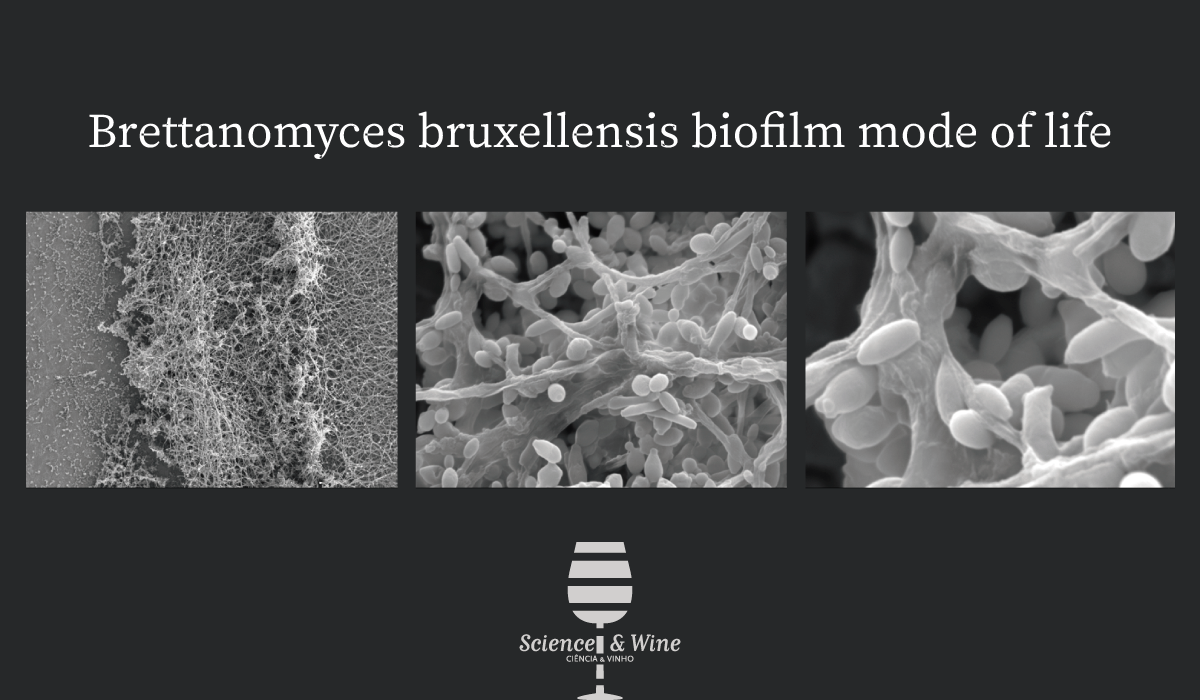
In the wine industry, one of the most feared spoilage microorganisms is the yeast Brettanomyces bruxellensis. This yeastis responsible for the production of volatile phenols and most importantly 4-ethylphenol, which contributes to undesirable aromas described as “Brett character”, leading to rejection by consumers and to heavy economic losses. This yeast can be found at several steps in the winemaking process due to its resistance to multiple stress conditions. The ability to form biofilm is a potential resistance strategy, although in the case of B. bruxellensis it has been given only little attention so far. Few studies demonstrated the ability of B. bruxellensis to adhere and form a biofilm-like, there is a lack of knowledge of these biofilm-like structures.
In this context, the purpose of our study was to study the biofilm structure by microscopic observations.
Brett’s biofilm structure
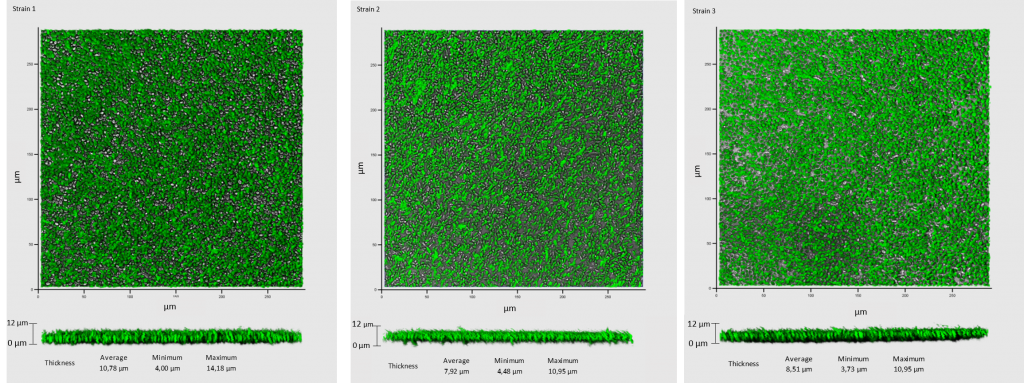
Seven day-aged biofilms in synthetic medium for some strains (IUVV collection) isolated from enological materials (i.e. from barrels, taps, pipes, transfer tanks) and/or wine from a winery were observed by Confocal Laser Scanning Microscopy (CLSM) to investigate biofilm characteristics. CLSM observations showed cellular layers covering the entire surface (Figure 2). An average thickness of 9.45 µm was measured (obtained from 50 measurements of random biofilm cuts of the representative views). Taken together, these data suggest that all the strains tested were able to develop in contact with a surface.
Although CLSM provided an overview of the cells adhered, additional SEM observations were necessary to demonstrate and validate characteristic structures of biofilm development. Observations for 7 days-aged biofilms developed on stainless-steel chips in synthetic medium revealed the presence of microcolonies containing cells embedded in ECS and filamentous cells possibly playing a role in their cohesion (Figure 3).

Chlamydospore-like structure, a new piece of B. bruxellensis morphotype
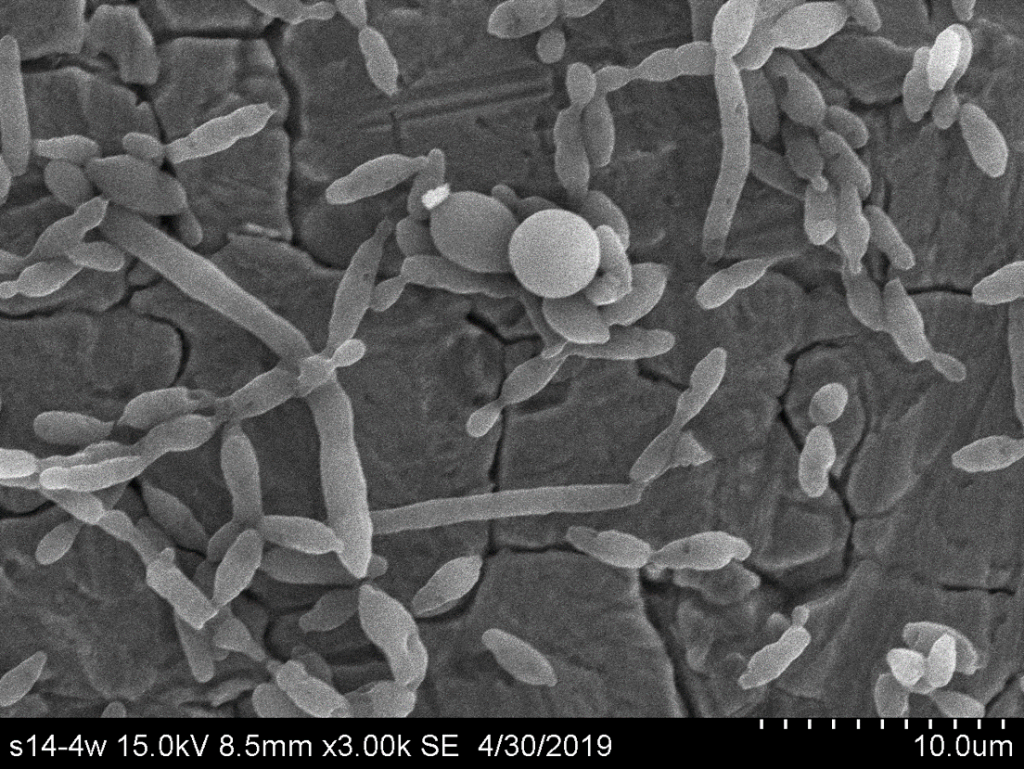
At the same time, 14 day-aged biofilms in wine were realized and microcolonies containing cells embedded in ECS and filamentous cells were also observed by SEM (Figure 4). Moreover, specific round, large and free shaped cells were observed (Figure 4). These structures are consistent with the definition of a chlamydospore, a morphological structure defined as larger than a yeast cell, highly refractile cells with thick walls derived from filamentous cells. Such characteristics were reported for the description of chlamydospore-like structures in C. albicans.
In this work, microscopic observations of biofilm structures have been performed to obtain better insight into the biofilm structure of B. bruxellensis. The both microscopy methods used highlight different points. CLSM allowed notably to gain information on the capacity to form biofilm and the thickness of the biofilm-like structure while SEM enable to observe easily different cell structures (i.e. cells, filaments, chlamydospores) and ECS. The 7 day-aged biofilms formed by the B. bruxellensis strains studied in this work had an average thickness of 9.45 µm which is rather thin compared to biofilms described for other yeast species. In this work, CLSM and SEM observations revealed the presence of several filamentous cells that appeared to start from the base of the biofilm and extend upward, suggesting the beginning of a multilayer structure.
Since B. bruxellensis is the major spoilage yeast of wine, it was crucial to enrich the information available on its capacity to form biofilms in enological environments. B. bruxellensis strains were able to form biofilms in synthetic medium but also in wine on stainless steel chip. Finally, microscopic observations in wine unexpectedly revealed the presence of “chlamydospore-like” structures that have never been observed for B. bruxellensis. Chlamydospores were described as forms of resistance in some fungi, however in yeast, their role was never clearly identified. So, future works should be carried out to determine the role of these “chlamydospore-like” structures for Brettamomyces yeast.
Sourced from the research article “New advances on the Brettanomyces bruxellensis biofilm mode of life” Lebleux Manon, Abdo Hany, Christian Coelho, Louise Basmaciyan, Warren Albertin, Julie Maupeu, Julie Laurent, Chloé Roullier-Gall, Hervé Alexandre, Michèle Guilloux-Benatier, Stéphanie Weidmann and Rousseaux Sandrine 318 (2020) 108464 International Journal of Food Microbiology https://doi.org/10.1016/j.ijfoodmicro.2019.108464
Correspondence to : Sandrine.rousseaux@u-bourgogne.fr

Stéphanie Weidmann-Desroche 
Sandrine Rousseaux 
Dr Stéphanie Weidmann-Desroche
Associate Professor at the University of Burgundy in Microbiology at the Faculty of Earth, Life and Environmental Sciences (UFR SVTE) and co-responsible of the international Master’s degree Microbiology and Physicochemistry for food and wine Processes (MP2).
She performs its research activities at the UMR PAM VAlMiS team (“Vin Aliment Microbiologie Stress”) in Dijon. Its main theme focuses on the stress response mechanisms of wine microorganisms, with particular attention to the involvement of sHsp-type stress proteins and the formation of biofilms.
https://www.umr-pam.fr/images/stories/pdf/Fiches_Individuelles/Fiche_CV_St%C3%A9phanie_WEIDMANN.pdf
http://www.ubfc.fr/master-mp2/
Lebleux M.*, Abdo H.*, Coehlo C., Basmaciyan L., Albertin W, Maupeu J., Laurent J., Roullier-Gall C., Alexandre H., Guilloux-Benatier M., Weidmann S., Rousseaux S. (2020) New advances on the Brettanomyces bruxellensis biofilm mode of life. International Journal of Food Microbiology. 318: 108464.
Lebleux M., Abdo H., Coehlo C., Basmaciyan L., Albertin W, Maupeu J., Laurent J., Roullier-Gall C., Alexandre H., Guilloux-Benatier M., Weidmann S., Rousseaux S. (2020) New advances on the Brettanomyces bruxellensis biofilm mode of life. International Journal of Food Microbiology. 318: 108464.
Coelho C., Gougeon R., Perepelkine L., Alexandre H., Guzzo J., Weidmann S. (2019)Chemical transfers occurring through Oenococcus oeni biofilm in different enological conditions. Frontiers in Nutrition 6: 95.
Weidmann S., Maitre M., Laurent J., Coucheney F., Rieu A., Guzzo J. (2017). Overproduction of the small heat shock protein Lo18 from Oenococcus oeni in Lactococcus lactis improves its stress tolerance. International Journal of Food Microbiology 247: 18-23.
Bastard A., Coelho C., Briandet R., Canette A., Gougeon R., Alexandre H., Guzzo J., Weidmann S. (2016). Effect of biofilm formation by Oenococcus oeni on malolactic fermentation and release of aromatic compounds in wine. Frontiers in Microbiology 7: 613.
Dr. Sandrine Rousseaux
Assistant professor in Viticulture and Microbiology, Vine pests at the Vine and Wine Institute (IUVV) of the University of Burgundy (uB) and responsible of a Master’s degree in Vine wine and Terroir.
She performs its research in the “Vin Aliment Microbiologie Stress” team at the Vine and Wine Institute (IUVV), Dijon. Her main thematic deals with the evolution of communities in different environments (soil, vine, must or wine but also in winery and cellar) using global approaches (independant culture methods) but also characterization and identification techniques at strain level. She study also different wine microorganisms (species of oenological interest or alteration).
http://www.umr-pam.fr/images/stories/pdf/Fiches_Individuelles/sandrine-rousseaux.pdf
https://www.researchgate.net/profile/Sandrine_Rousseaux
Longin C, Degueurce C, Julliat F, Guilloux-Benatier M, Rousseaux S, Alexandre H (2016) Efficiency of population-dependent sulfite against Brettanomyces bruxellensis in red wine. International Journal of Food Microbiology 89 : 620-630. https://doi.org/10.1016/j.foodres.2016.09.019
Longin C, Julliat F, Serpaggi V, Maupeu J, Bourdon G, Rousseaux S, Guilloux-Benatier M, Alexandre H (2016) Evaluation of three Brettanomyces qPCR commercial kits: results from an interlaboratory study. OenoOne 50: 223-230 https://doi.org/10.20870/oeno-one.2016.50.4.1274
Lebleux M, Abdo H, Coelho C, Basmaciyan L, Albertin W, Maupeu J, Laurent J, Roullier-Gall C, Alexandre H, Guilloux-Benatier M, Weidmann S, Rousseaux S. New advances on the Brettanomyces bruxellensis biofilm mode of life. Int J Food Microbiol https://doi.org/10.1016/j.ijfoodmicro.2019.108464