By Luciana Mosca and Alberto Macone
RSV is a naturally occurring compound produced by plants in response to biotic and abiotic stress, including pathogenic invasion, physical trauma and UV irradiation and occurs in two isomeric forms, cis- and trans-RSV, the latter being dominant in terms of prevalence and biological activity.
RSV can be found as a glucoside derivative in many plant foods (red grapes, berries, peanuts, soy, etc.), while in red wine it is mainly present as an aglycone derivative. Resveratrol content in red wine comes from grapes (Vitaceae), being the skin, seeds, petioles, and woody parts the richest sources. Even though the direct free radical scavenging activity of resveratrol is relatively poor, in vitro, ex-vivo and in vivo experiments show that its antioxidant properties are likely due to its effect as a gene regulator. Thus, it is not surprising that this molecule may affect many cellular processes including metabolism, stress resistance, cell survival, aging inflammation and immune response. However, although commonly used as a dietary supplement, and despite the number of beneficial effects described over the years, there is little clinical evidence that RSV could be an effective therapeutic agent in humans. Similarly, very little is known about the effects of RSV derivatives (synthetic and natural analogues as well as adducts, derivatives, and conjugates) on human health and only a few studies have been set up to determine the levels of the other stilbenes in wines, with the exception of the cis-RSV, and their 3-O-β-glucosides.
Recently we have focused our attention on resveratrol photo-oxidation products. It is known that, when exposed to UV radiation, trans-RSV isomerizes to cis-RSV. The latter can subsequently undergo electrocyclization leading to the formation of THP (Figure 1).
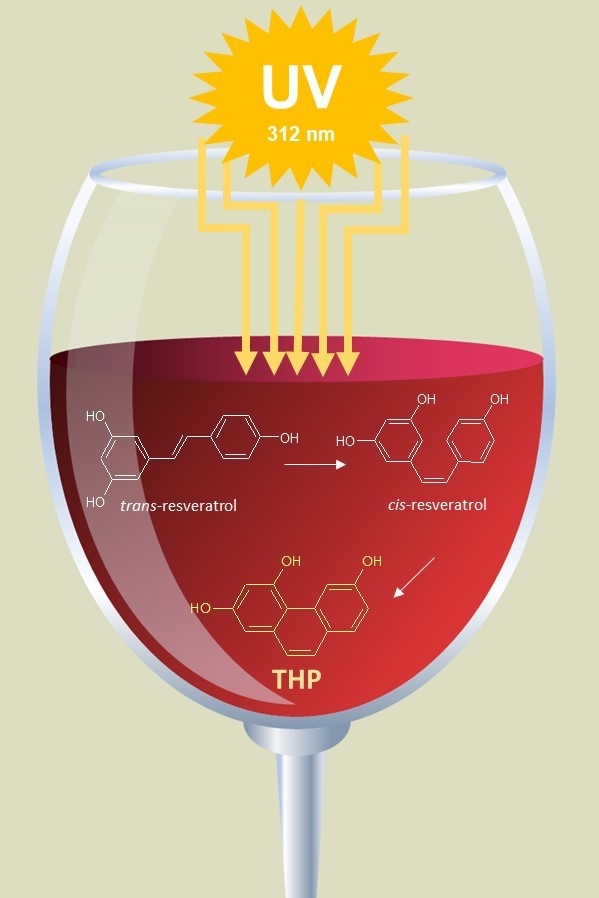
To the best of our knowledge, THP has not yet been detected in RSV rich foods or in beverages such as red wine. Considering that red wine is the main source of trans-RSV, and the cis-isomer can be formed in this matrix as the result of UV-light induced isomerization, it is reasonable to hypothesize the presence of THP as well.
In our laboratory, at Sapienza University, we set up a simple procedure for the simultaneous qualitative and quantitative analysis of trans-RSV, cis-RSV and THP in red wine, before and after UV-light exposure. Being these molecules sparingly soluble in water, we performed a liquid-liquid extraction using ethyl-acetate. The organic extracts were then submitted to a derivatization procedure in order to make RSV isomers and THP suitable to the subsequent gas chromatographic-mass spectrometric analysis.
In setting up and validating the analytical method, we had to solve two different critical issues: i) cis-RSV and THP are not commercially available (this represents one of the major limits to the studies on these molecules); ii) due to the complexity and the natural variability of wine chemical composition, the validation of the analytical method should be done on an artificial matrix (synthetic wine) that can resemble, as much as possible, a generic red wine.
To solve this latter issue, we prepared and used for the validation of the analytical method a synthetic red wine consisting in a water solution containing 13% ethyl alcohol, 1% glycerol and a variety of acids, such as succinate, malate and tartrate (making up an additional 0.8%) at a final pH of 3.3.
Concerning the issue arising from the lack of commercially available analytical standards, cis-RSV can be easily prepared by exposing trans-RSV dissolved in isopropanol or methanol to sunlight or UV light. However, in these conditions, the subsequent rate of conversion of cis-RSV to THP is very low. We were able to show that when the UV irradiation is performed in ethanol-water solution (13:87 v/v), higher rate of conversion of cis-RSV to THP can be achieved. This procedure yields cis-RSV and THP directly from trans-RSV at concentrations suitable to setting-up the analytical method. Thus, by varying the exposure time to UV light, it is possible to fine-tune the relative concentrations of RSV isomers and THP.
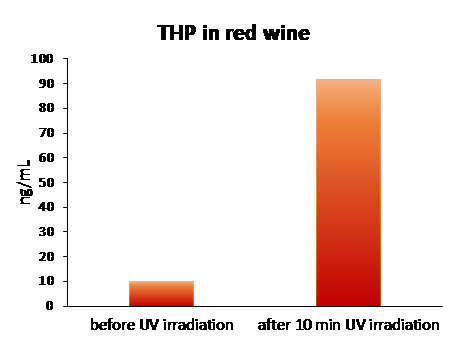
Once developed and fully validated, the analytical method was then tested on a selection of commercial and homemade red wines from different Italian regions, grape varieties and vintages showing for the very first time the presence of THP in this matrix.
These wines were analyzed before and after 10 min of UV irradiation. As expected, UV light induces trans-RSV isomerization followed by cis-RSV cyclization to THP. After only 10 min of UV light treatment, a mean 9.35-fold increase of THP can be observed (Figure 2). The studies on THP are in the early stages and, at present, the potential effects of this molecule on human health are not yet known. Being a polycyclic aromatic hydrocarbon, THP could indeed exert cytotoxic and genotoxic effects. It has been demonstrated that THP can induce DNA damage through a pro-oxidant mechanism on Caulobacter crescentus model system, even at sub-micromolar concentrations. On the other hand, due to the extended delocalization and conjugation of the π electrons over the entire molecule, THP displays a significantly increased antioxidant activity compared to RSV with potential benefits on human health.
Whatever the effect of THP on human health, it becomes important to establish its actual presence not only in RSV-containing food and beverages but even in cosmetics that, by their nature, are subject to UV radiation.

Dr Alberto Macone: master degree in Biological Sciences, residency in Clinical Pathology and Ph.D. in Biochemistry. Currently, he is assistant professor at the Department of Biochemical Sciences “A. Rossi Fanelli, Sapienza University of Rome (Italy) where he is head of the Metabolomics Lab. His research activity is mainly focused on metabolome analysis (metabolite target analysis, profiling and fingerprinting) through the development and validation of chromatographic and mass spectrometric methods for the study of metabolic profiles associated with specific cellular processes, physiological and pathological, in different biological systems. Part of the research work is dedicated to plant metabolomics (biochemical characterization of plant pathogen interaction) and to the application of metabolomics to food quality and safety.
mail: alberto.macone@uniroma1.it
ORCID ID: 0000-0003-0455-1400
ResearcherID: AAB-2570-2019

Dr. Luciana Mosca: master degree in Pharmacy, PhD in Biochemistry, residency in Human Nutrition and master in Clinical Research. Currently she is Assistant Professor at the Department of Biochemical Sciences, Sapienza University of Rome. She has a longstanding experience in the field of oxidative stress and free radicals. She focuses on the mechanisms behind oxidative stress triggers of cell death in neurodegenerative diseases and on the identification of natural substances able to counteract this phenomenon. Particularly, bioactive polyphenols isolated from foods rich in antioxidants, from spices or from medicinal plants are the main object of her interest. She is author of more than 60 papers on peer reviewed international journals and of 5 book chapters. She holds two patents as inventor. She teaches biochemistry and nutritional biochemistry in pre- and post-graduation courses.
Mail: luciana.mosca@uniroma1.it
ORCID ID: 0000-0003-2748-9021
Researcher ID: K-8101-2015
References
- “Gas chromatographic-mass spectrometric method for the simultaneous determination of resveratrol isomers and 2,4,6-trihydroxyphenanthrene in red wines exposed to uv-light”.
- Francioso A, Laštovičková L, Mosca L, Boffi A, Bonamore A, Macone A. J Agric Food Chem. 2019, 67,11752-11757. doi: 10.1021/acs.jafc.9b05992.
- “Isolation and identification of 2,4,6-trihydroxyphenanthrene as a byproduct of trans-resveratrol photochemical isomerization and electrocyclization”.
Francioso A, Boffi A, Villani C, Manzi L, D’Erme M, Macone A, Mosca L. J Org Chem. 2014, 79, 9381-4. doi: 10.1021/jo501405m. - “2,4,6-Trihydroxyphenanthrene, a trans-resveratrol photoreaction byproduct: first evidences of genotoxic risk”.
Francioso A, Mosca L, Menéndez-Perdomo I.M, Fanelli S, Fontana M, D’Erme M, Fuentes-Leon F, Sanchez-Lamar A. Phytochem. Lett. 2019, 30, 362-366. - “Synthesis and antioxidant activity of hydroxylated phenanthrenes as cis-restricted resveratrol analogues”.
Ding D.J, Cao, X.Y, Dai, F, Li X.Z, Liu G.Y, Lin D, Fu X, Jin X.L, Zhou B. Food Chem. 2012, 135, 1011-9. doi: 10.1016/j.foodchem.2012.05.074.

