By Alba Ramos-Pineda, Ignacio García-Estévez, M. Teresa Escribano-Bailón
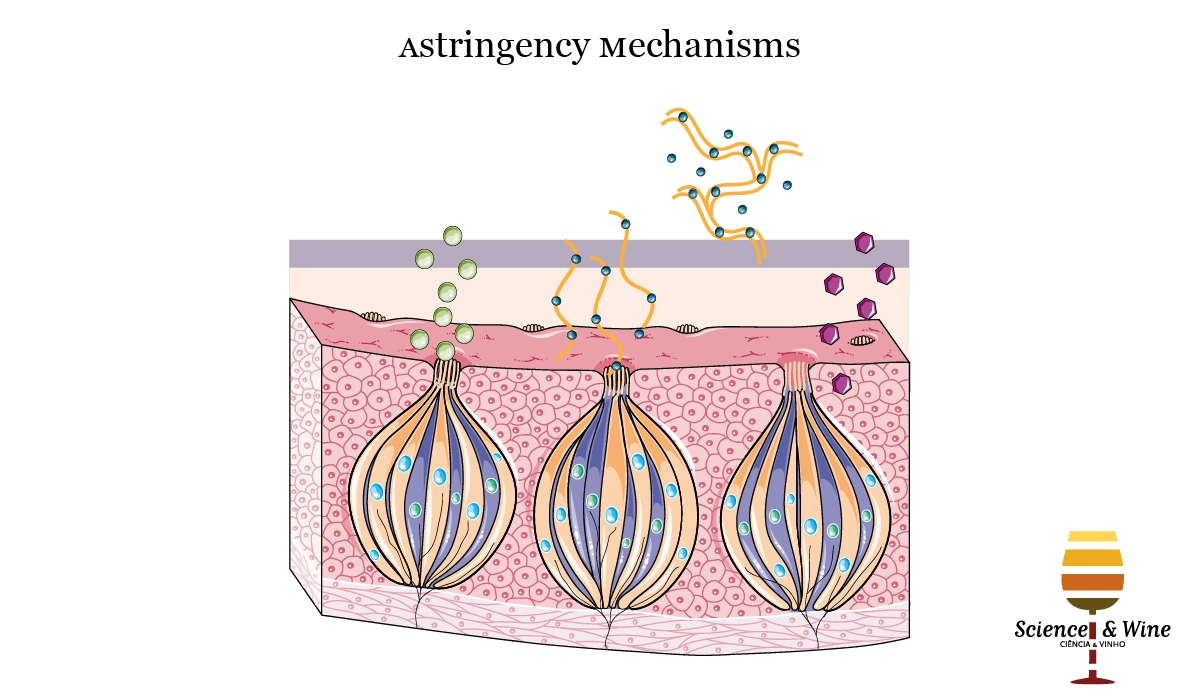
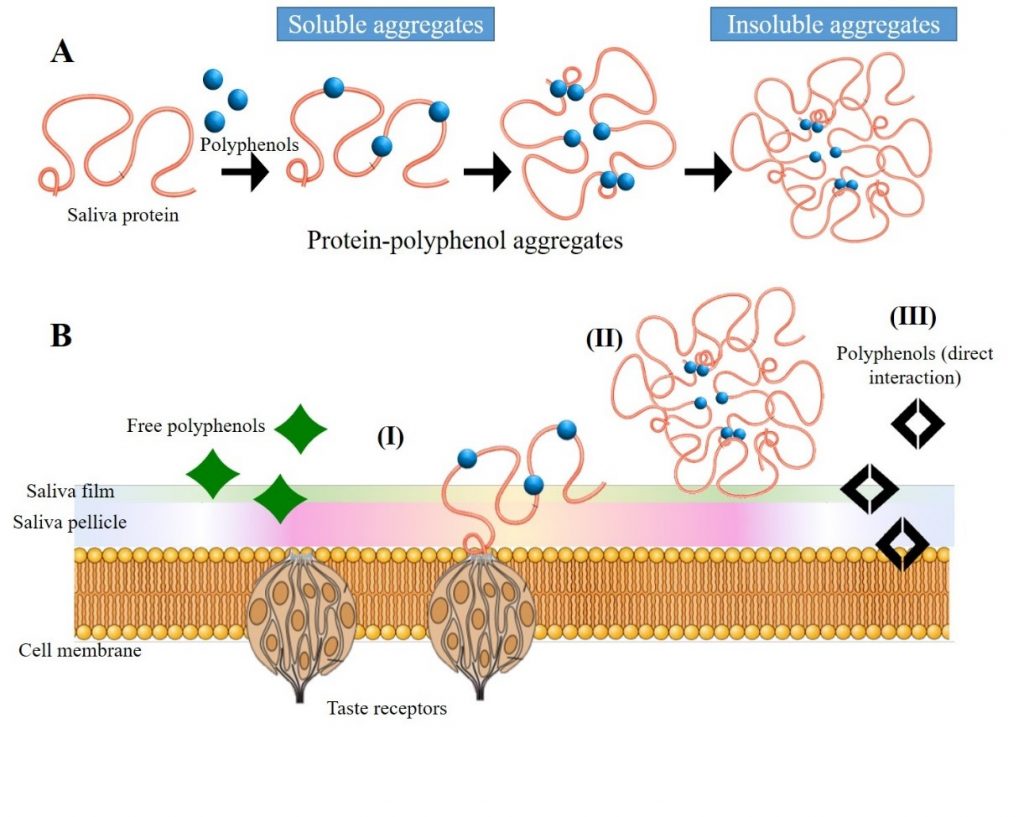
The oral sensation called astringency is commonly described as drying, roughing and puckering in the mouth epithelia (Gawel et al., 2000). Although the bases of the astringency mechanism are not well understood yet, it is known to be engendered by different classes of astringent compounds and, among them, polyphenols (Bajec and Pickering 2008, Joslyn and Goldstein 1964). Since some polyphenols are able to bind salivary proteins, namely PRPs, they can form insoluble tannin-protein precipitates in the mouth, causing a loss of lubrication and increased friction in the oral cavity, which would explain its astringency (Baxter et al., 1997). This mechanism is thought to be the main responsible of wine astringency. Among polyphenols, flavanols, flavonols and anthocyanins have shown to be able to interact with PRPs and, therefore, they can be considered as astringent compounds. In fact, flavonol glycosides produce a mouth-drying and mouth-coating sensation at very low threshold concentrations (Scharbert and Hofmann, 2005). However, not all astringents cause salivary protein precipitation, evidencing there must be other mechanisms implicated in astringency development. Indeed, other approaches have suggested that astringency could be engendered by activation of specific taste receptors (Tachibana et al., 2004) or even by direct interactions between tannins and oral epithelial cells (Payne et al., 2009). Gibbins and Carpenter (2013) proposed that the complex sensation of astringency could involve multiple mechanisms occurring simultaneously (see Fig. 1): aggregation of salivary proteins, salivary film disruption, decrease in salivary lubrication, receptors exposure and mechanoreceptors stimulation in the oral mucosal epithelium. Nevertheless, since there are no conclusive results, the scientific community is still discussing the different proposed mechanisms that explain this complex phenomenon.
It is widely acknowledged that the astringent sensation can be modulated by several factors, like the presence of acid, sugars, ethanol, and others. Different studies have revealed that several polysaccharide families are able to interact with tannins, so they could reduce astringency by limitation of available proanthocyanidins (García-Estévez et al., 2017). Thus, polysaccharides could be used to improve astringency with an increase of the wine sweetness and roundness. Among wine polysaccharides, mannoproteins represent ca. 35% of total wine polysaccharides (Vidal et al., 2003). Mannoproteins are glycoproteins from the cell wall of the Saccharomyces cerevisiae that can be excreted to the wine during alcoholic fermentation or be released to the wine during yeast autolysis (Ribereau-Gayon et al., 2000). They are considered as protective colloids that protect wine from protein haze (Dupin et al., 2000) and that can prevent tartrate precipitation (Moine-Ledoux and Dubourdieu 2002). The interest of using mannoproteins in winemaking has gradually increased during the last years and different commercial mannoproteins formulates have been developed in order to modify the phenolic composition and the organoleptic properties of wines (Guadalupe et al., 2010).
A recent study has evaluated the effect of the addition of yeast mannoproteins (MP) on the interaction between a flavonol glucoside (quercetin 3-O-glucoside) and human salivary proteins, combining sensory analysis and analytical techniques such as quenching fluorescence, dynamic light scattering (DLS) and isothermal titration calorimetry (ITC). Sensory analysis confirmed the ability of mannoproteins to decrease the astringency elicited by the flavonol. Results obtained with the different analytical techniques indicate the existence of interactions between mannoproteins and flavonols but also between mannoproteins and salivary proteins, suggesting a possible formation of protein/polyphenol/polysaccharide ternary complex that probably affects the astringency perception (Ramos-Pineda et al., 2018).
Those interested in a longer length report can download the working paper at:
https://www.sciencedirect.com/science/article/pii/S0308814618307593

Food Quality Research Team. Grupo de Investigación en Polifenoles (GIP) – Department of Analytical Chemistry, Nutrition and Food Sciences – University of Salamanca (USAL), Salamanca, Spain.
Our research projects are focused on the study of the role of phenolic compounds on quality and stability of food products. The main research topic is related to the impact of the phenolic maturity of red grapes on sensory quality of red wines, namely on color and astringency properties. Recent works aimed to go deepen on the study of the mechanism for astringency development through the use of physical-chemical studies, in order to provide the wine industry (both wineries and oenological industries) with basic knowledge about this complex sensation.
Furthermore, our studies are also related to the analysis and characterization of phenolic composition (mainly flavonoids and phenolic acids) of different food and plant matrices.
Research Group: (Left to right) Rebeca Ferras Charro, Dr Elvira Manjón Pérez, Dr. Montserrat Dueñas Patón, Dr. M. Teresa Escribano Bailón, Dr Ignacio García Estévez, Dr Cristina Alcalde Eon, Alba M. Ramos Pineda, Ana Navarro Martínez.
References:
- Bajec, M.R. and Pickering, G.J. (2008). Astringency: mechanisms and perception. Critical Reviews in Food Science and Nutrition 48:858-875.
- Baxter, N.J., Lilley, T.H., Haslam, E. and Williamson, M.P. (1997). Multiple interactions between polyphenols and a salivary proline-rich protein repeat result in complexation and precipitation. Biochemistry 36: 5566-5577.
- Dupin, I. V. S., Stockdale, V. J., Williams, P. J., Jones, G. P., Markides, A. J. and Waters, E. J. (2000). Saccharomyces cerevisiae mannoproteins that protect wine from protein haze: evaluation of extraction methods and immunolocalization. Journal of Agricultural and Food Chemistry 48: 1086-1095.
- García-Estévez, I., Ramos-Pineda, A.M. and Escribano-Bailón, M.T. (2018) Interactions between wine phenolic compounds and human saliva in astringency perception. Food and Function 9: 1294-1309.
- Gawel, R., Oberholster, A. and Leigh Francis, I. (2000). A ‘Mouth-feel Wheel’: terminology for communicating the mouth-feel characteristics of red wine. Australian Journal of Grape and Wine Research 6:203–207.
- Gibbins, H.L. and Carpenter, G.H. (2013). Alternative mechanisms of astringency–what is the role of saliva? Journal of Texture Studies 44: 364-375.
- Guadalupe, Z., Martínez, L. and Ayestarán, B. (2010) Yeast mannoproteins in red winemaking: effect on polysaccharide, polyphenolic, and color composition. American Journal of Enology and Viticulture 61: 191-200.
- Joslyn, M.A. and Goldstein, J.L. (1964). Astringency of fruits and fruit products in relation to phenolic content. Advances in Food Research 13: 179-217.
- Ma, W., Guo, A., Zhang, Y., Wang, H., Liu, Y. and Li, H. (2014). A review on astringency and bitterness perception of tannins in wine. Trends Food Sci. Technol. 40:6-19.
- Moine-Ledoux, V. and Dubourdieu, D. (2002). Rôle des mannoprotéines de levures vis-à-vis de la stabilisation tartrique des vins. Bulletin de l’O.I.V, 75: 471-482.
- Payne, C., Bowyer, P.K., Herderich, M. and Bastian, S.E. (2009). Interaction of astringent grape seed procyanidins with oral epithelial cells. Food Chemistry 115: 551-557.
- Ramos-Pineda, A. M., García-Estévez, I., Dueñas, M., and Escribano-Bailón, M. T. (2018). Effect of the addition of mannoproteins on the interaction between wine flavonols and salivary proteins. Food Chemistry 264:226-232.
- Ribéreau-Gayon, P., Dubourdieu, D., Donèche, B. and Lonvaud, A. (2000). Handbook of Enology. Vol. 1. The Microbiology of Wine and Vinifications, 1st ed., Wiley: Chichester, UK, 454 pp.
- Scharbert, S., & Hofmann, T. (2005). Molecular definition of black tea taste by means of quantitative studies, taste reconstitution, and omission experiments. Journal of Agricultural and Food Chemistry 53:5377–5384.
- Tachibana, H., Koga, K., Fujimura, Y. and Yamada, K. (2004). A receptor for green tea polyphenol EGCG. Nature Structural and Molecular Biology11: 380-381.
- Vidal, S., Williams, P., Doco, T., Moutounet, M., & Pellerin, P. (2003). The polysaccharides of red wine: total fractionation and characterization. Carbohydrate Polymers, 54, 439-447.