By Simone Vincenzi (Mattia Di Gaspero, Paolo Ruzza, Rohanah Hussain, Barbara Biondi, Giuliano Siligardi and Andrea Curioni)
In white wines, even if present at low concentration, proteins have considerable importance because they greatly affect wine stability. The major proteins implicated in haze formation in wine belong to the grape-derived pathogenesis-related proteins (PRP) which withstand the vinification process and persist in the wine after bottling. Current model of haze formation suggests the unfolding of these proteins, mainly due to inappropriate storage conditions, with the exposition of the hydrophobic sites, which make the proteins susceptible to aggregation. Then, the so-formed aggregates gradually grow up through processes of cross-linking with other wine components, such as polyphenols. Polyphenols are important constituent of wines and this family of compounds comprises tannins and other compounds of lower molecular weight. While the ability to bind proteins is well known for tannins, which are polymers of several catechin units (fig. 1) less information is available on the interaction with proteins of polyphenols with a lower molecular weight. To fill this lack of information, the effect of catechin dimers (procyanidins B1 and B2), which are the more represented procyanidins in white wines, and flavonols (quercetin and rutin, fig. 1) were considered as well as a total white wine tannin extract and tannic acid (a polymeric tannin not naturally present in wines, fig.1). All these compounds were tested for their interactions with the main wine protein, Vitis Vinifera Thaumatin-Like-1 protein (VVTL1) which was purified from wine.
To study these interactions, Synchrotron Radiation Circular Dichroism (SRCD) was used. Among other things, this technique allows to follow fine variations in the protein structure and conformation in the presence of other molecules, thus giving information on the occurrence of interactions. The conformational properties of the wine protein alone were studied and then compared to that resulting from the presence of the different polyphenols tested. In addition, considering the importance of the protein denaturation for haze formation in wines, the effect of such interactions with polyphenols on the stability of the protein was studied by both thermal and UV-photo induced denaturation experiments.
The SRCD spectra of the different samples (VVTL1 protein alone and in the presence of the different polyphenols) were recorded in both the far and near UV regions.
With the far-UV system it is possible to detect variations in the ordered structures present in the protein structure, such as α-helices and β-sheets. The spectra of VVTL1 recorded in the presence of different polyphenols showed little differences in comparison to that of VVTL1 alone.
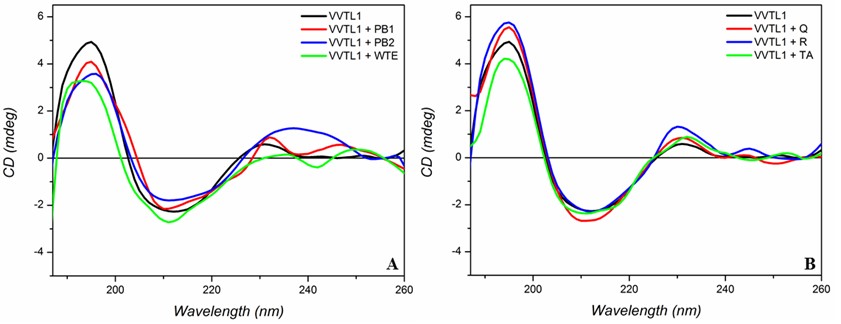
In particular, PB1, PB2, WTE and TA decreased the intensity of the positive band at 195 nm (Figure 2), while with Q and R the intensity of this band increased. No effects were observed on the negative band at 210 nm. According to these results it is possible to estimate that in presence of polyphenols the β-sheet content of VVTL1 protein decreases less than the 10%. In addition, the strong modification of the band at 230-250 nm, which indicates the contribute of the aromatic chromophore transition, suggests that the interaction likely involves the aromatic residues of the protein.
Spectra in the near-UV region, which are related to the protein tertiary structure, confirmed the effects of the different polyphenols on VVTL1 protein. In particular, PB1, PB2, WTE and Q decreased the intensity of both negative band and shoulder at 290 nm and 300 nm, respectively. However, in presence of rutin (R), the near-UV CD spectrum of VVTL1 was profoundly modified, indicating a more pronounced interaction of this polyphenol with the protein. This may be attributable to the presence of the glycoside moiety in rutin that could increase hydrogen bonding between polyphenol and protein favouring its interaction with VVTL1 protein.
Once determined that the tested polyphenols influenced the protein structure which is indicative of their interaction, the effects of the same polyphenols on the thermal stability of VVTL1 protein were assessed. By treating the protein alone with increasing temperatures (from 5 to 70°C), a drastic change in the SRCD spectrum was detectable starting from temperatures above 54°C were an increase in the unordered structure was observed, indicating protein denaturation. A very similar behavior was detected also in the presence of the tested polyphenols, but in this case the loss of secondary structure content occurred at a temperature different from that determined for the VVTL1 alone, indicating that the interaction with polyphenol tends to change the thermal stability of the wine protein. The stability was increased in the presence of PB1, PB2, and WTE, but decreased when TA, Q, and R were present.
In conclusion, it has been demonstrated that not only polymeric tannins, but also smaller polyphenolic compounds which are present in white wines are able to interact with at least one component (VVTL1) of the two main protein classes (TLPs and Chitinases) found in wines and involved in protein haze formation. However, the most important finding is that this interaction seems to lead to a change in the heat-stability of the protein, which is of great importance for the mechanism of protein aggregation and hazing in white wines. Therefore, these facts have to be taken into account when studying the reasons for wine hazing, because the whole complex matrix of the wine have the ability to modulate protein behaviour.

Simone Vincenzi is professor of Food Science and Enology at Padua University (Italy) and carries out his research activity in the Campus of Conegliano, in the heart of Prosecco region. His expertise is focused on wine stability and fining. He published more than 50 scientific papers and he is co-editor of the Italian Journal of Food Science.

