By Letizia Bresciani
Epidemiological evidence has associated phenolic-rich foods with the prevention of several chronic pathologies, including cardiovascular diseases, diabetes, neurodegenerative diseases and some types of cancer (Zanotti et al,. 2015, Tresserra-Rimbau et al,. 2014, Liu et al,. 2014, Rothwell et al,. 2017). Due to these claimed, but not well clarified effects, the interest of consumers in polyphenol-rich foods increased exponentially in the last decades, with the consequent injection into the global market of several new products rich in phenolic compounds. Obviously, the claimed effects of any given product must be unequivocally demonstrated, and the potential benefits are strictly related to absorption, bioavailability and metabolism of the putatively bioactive compounds contained in it (Del Rio et al,. 2013, Rodriguez-Mateos et al,. 2014). Red wine production generates an important amount of grape by-products. Recent European policies encourage the re-utilization of these industry by-products, which may represent a readily available, low cost, innovative functional ingredients, exploitable to increase the bioactive fraction of a wide range of food products or beverages. Grape pomace is the major by-product of wine and grape juice industry and it represents a rich source of polyphenols, including anthocyanins and flavan-3-ols, in the form of monomers and oligomers (commonly known as tannins), together with flavonols, flavanones, phenolic acids and their derivatives, stilbenes and lignans. In the papers here discussed, a red grape pomace drink rich in polyphenols has been characterized and comprehensive pharmacokinetic and excretive profiles of phenolic metabolites after the acute administration of the drink (Castello et al,. 2018) have been performed. Moreover, the acute effects of the drink on glucose/insulin and triglyceride responses to a standard meal in healthy individuals and the relationship between plasma levels of phenolic metabolites and metabolic parameters (Costabile et al,. 2018) have been investigated.
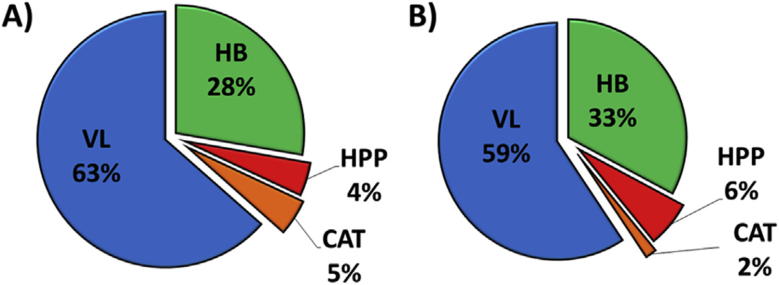
In the first work, a total of 28 phenolic metabolites were quantified in the blood plasma of ten volunteers who consumed the red grape pomace drink. Phenyl-γ-valerolactones (VL) were the most abundant class of phenolic metabolites in circulation (63%) (Figure 1A). Additionally, 35 polyphenol metabolites were quantified in urine collected within 48 h after drink consumption. As reported for plasma, phenyl-γ-valerolactones (VL) were the main class of excreted phenolic metabolites (59%), followed by simple phenols and hydroxybenzoic acids (HB), hydroxyphenylpropionic and hydroxycinnamic acids (HPP) and (epi)catechins (CAT) (Figure 1B). Both in plasma and in urine, the phase II conjugates of 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone were the highest contributors.
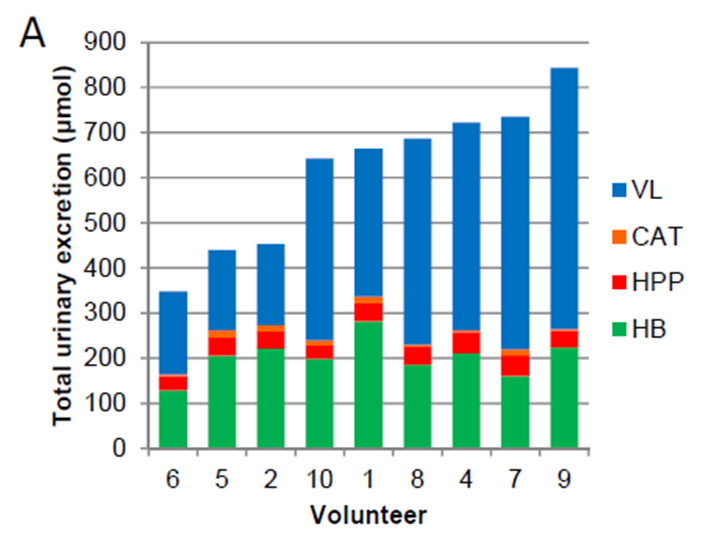
A high inter-individual variability was demonstrated, both in plasma and in urine samples, and different patterns of circulating metabolites were unraveled. The variance (CV%) for each urinary excreted metabolite ranged from 20 to 167%, with a mean CV of 52%. Considering all the metabolite classes, the excreted phenolic metabolites varied between 348 and 844 μmol in 48h (Fig. 4A-B).
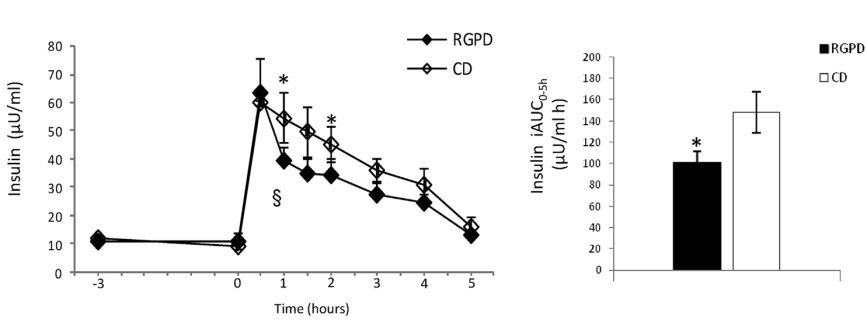
The potential health benefits of drink intake was evaluated on 12 healthy men in a second study. All participants consumed a drink rich in polyphenols or a control drink (no polyphenols), followed, after 3h, by a standard meal (960 kcal, 18% protein, 30% fat, 52% carbohydrate). Glycemic and triglyceride post-meal responses were similar between the test and the control drink. In contrast, postprandial insulin levels, and, consistently, the incremental area (iAUC0-5h) were significantly lower after red grape pomace drink than after control drink consumption (Figure 3).
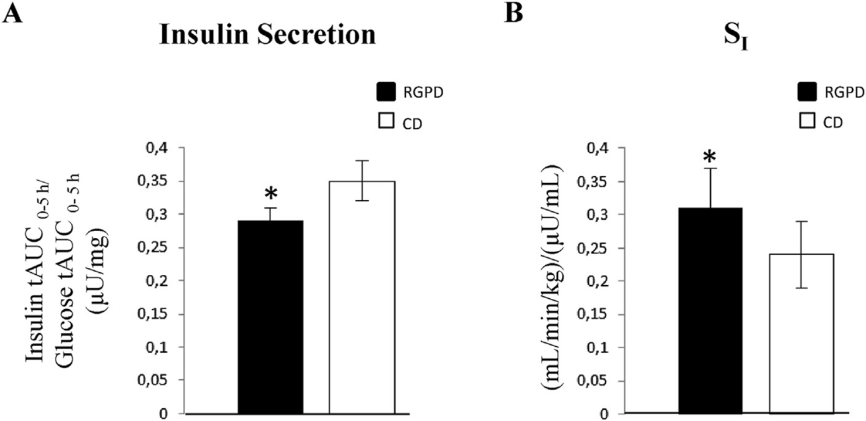
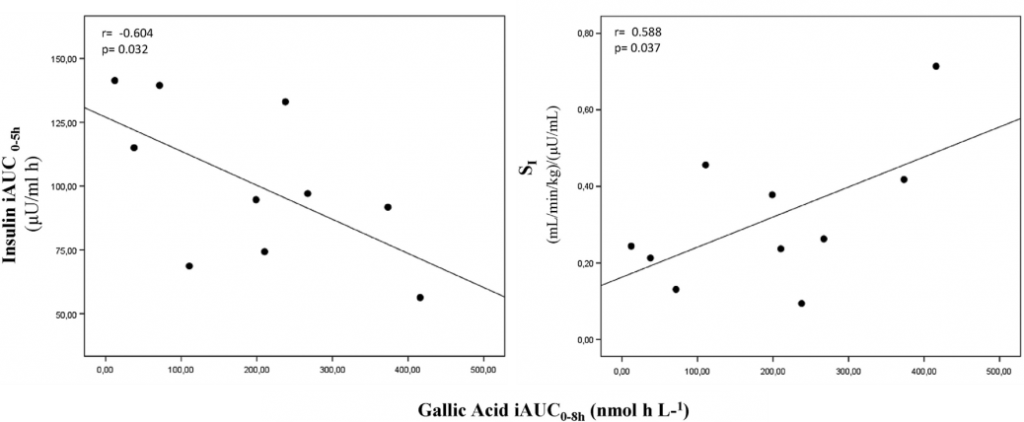
Insulin secretion index, calculated over 5h after the standard meal, was significantly lower (-18%), and insulin sensitivity (SI) index, significantly higher (36%) after red grape pomace drink consumption (Figure 4) with respect to control. Finally, a linear correlation analysis showed that, among the many circulating phenolic metabolites, gallic acid inversely correlated with insulin response and, quite expectedly, positively with insulin sensitivity, indicating gallic acid as the best predictor of the insulin sensitivity index, followed by epicatechin-glucuronide and dihydrocaffeic acid-sulphate. No other correlation reached the statistical significance.
In conclusion, these two works demonstrate that the intake of a drink based on red grape pomace can deliver significant amounts of different phenolic metabolites to the human system. However, more attention should be addressed to inter-individual variability, regarding both the production of gut microbial-derived metabolites and the phase II metabolic step. Moreover, besides the acute nature and the small sample size of the second study, polyphenols from red grape pomace drink have been shown to improve insulin sensitivity, an effect likely mediated by the increase in plasma levels of gallic acid. The use of by-products originating from food production as potentially beneficial ingredients represents a very wise strategy both in terms of public health and circular economy.

Dr. Letizia Bresciani: Master’s degree in food science and Technology (2011) and PhD in Food Science (2016) at the University of Parma. Currently, she is a Post-Doctoral Research Fellow at The Laboratory of Phytochemicals in Physiology, Human Nutrition Unit, Department of Veterinary Science of the University of Parma (Italy), led by Prof. Daniele Del Rio. Her expertise includes characterization of phenolic compounds from vegetables and investigation of pharmacokinetics, absorption, metabolism, bioavailability and bioactivity of human and microbial phenolic metabolites, using principally in vivo models.
References:
- Castello, F., Costabile, G., Bresciani, L., Tassotti, M., Naviglio, D., Luongo, D., Ciciola, P., Vitale, M., Vetrani, C., Galaverna, G., Brighenti, F., Giacco, R., Del Rio, D. & Mena, P. (2018). Bioavailability and pharmacokinetic profile of grape pomace phenolic compounds in humans. Arch Biochem Biophys, 646, 1-9.
- Costabile, G., Vitale, M., Luongo, D., Naviglio, D., Vetrani, C., Ciciola, P., Tura, A., Castello, F., Mena, P., Del Rio, D., Capaldo, B., Rivellese, A. A., Riccardi, G. & Giacco, R. (2018). Grape pomace polyphenols improve insulin response to a standard meal in healthy individuals: A pilot study. Clinical Nutrition.
- Del Rio, D., Rodriguez-Mateos, A., Spencer, J. P., Tognolini, M., Borges, G. & Crozier, A. (2013). Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal, 18, 1818-92.
- Liu, Y. J., Zhan, J., Liu, X. L., Wang, Y., Ji, J. & He, Q. Q. (2014). Dietary flavonoids intake and risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Clinical Nutrition, 33, 59-63.
- Rodriguez-Mateos, A., Del Pino-Garcia, R., George, T. W., Vidal-Diez, A., Heiss, C. & Spencer, J. P. (2014). Impact of processing on the bioavailability and vascular effects of blueberry (poly)phenols. Mol Nutr Food Res, 58, 1952-61.
- Rothwell, J. A., Knaze, V. & Zamora-Ros, R. (2017). Polyphenols: dietary assessment and role in the prevention of cancers. Curr Opin Clin Nutr Metab Care, 20, 512-521.
- Tresserra-Rimbau, A., Rimm, E. B., Medina-Remón, A., Martínez-González, M. A., de la Torre, R., Corella, D., Salas-Salvadó, J., Gómez-Gracia, E., Lapetra, J., Arós, F., Fiol, M., Ros, E., Serra-Majem, L., Pintó, X., Saez, G. T., Basora, J., Sorlí, J. V., Martínez, J. A., Vinyoles, E., Ruiz-Gutiérrez, V., Estruch, R. & Lamuela-Raventós, R. M. (2014). Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutrition, Metabolism and Cardiovascular Diseases, 24, 639-647.
- Zanotti, I., Dall’Asta, M., Mena, P., Mele, L., Bruni, R., Ray, S. & Del Rio, D. (2015). Atheroprotective effects of (poly)phenols: a focus on cell cholesterol metabolism. Food Funct, 6, 13-31.

