By Alba M. Ramos-Pineda, Ignacio García-Estévez, Montserrat Dueñas, M. Teresa Escribano-Bailón
Astringency is usually defined as the complex of sensations due to shrinking, drawing or puckering of the mouth epithelium (ASTM, 2004). Although the bases of this mechanism are not well understood yet, it is broadly assumed the ability of food tannins to interact with some salivary constituents, mainly salivary proteins, resulting in the formation of protein-tannin aggregates (de Freitas & Mateus, 2012, Rinaldi, Gambuti, & Moio, 2012), which, in turn, causes a decrease in salivary lubrication (Canon et al., 2013).
Salivary proteins have been classified into six groups attending to their structure and characteristics, namely, basic proline-rich proteins, acidic proline-rich proteins, glycosylated proline-rich proteins, statherin, histatins and cystatins (Castagnola, Cabras, Vitali, Sanna, & Messana, 2011; Soares et al., 2011). Among salivary proteins, proline-rich proteins are particularly effective in complexing tannins (Canon et al., 2015). More than 11 human basic proline-rich proteins and five acidic proline-rich proteins isoforms have been identified and the total proline-rich proteins represent >60% by weigh of the total salivary proteome (Inzitari et al., 2005; Messana et al., 2004). The main function proposed for the glycosylated proline-rich proteins is to act as lubricants, whereas proline-rich proteins are associated with tannin precipitation (Oppenheim et al., 2007). In contrast to proline-rich proteins, there are minor structural differences among the acidic proline-rich proteins and their predominant role proposed is related to mineral homeostasis and tooth integrity preservation (Oppenheim et al., 2007).
As aforementioned, the interaction between phenolic compounds and salivary proteins (mainly proline-rich proteins) has been described as key process to explain the astringency perception, with or without precipitation of the tannin-protein complexes (Cala et al., 2012; Ferrer-Gallego et al., 2017). However, studies carried out up till the present have not yet explored the different ability of salivary proteins fractions to interact with flavanols when they are isolated or mixed with other salivary proteins fractions, which could help for unraveling the astringency process.
In this context, this research aimed to study the effect of the coexistence of basic proline-rich proteins and acidic proline-rich proteins salivary fractions on the interaction with flavanols towards the interaction between the same flavanols with these fractions assayed individually, using techniques such as HPLC-DAD, DLS and MALDI-TOF.

To investigate the existence of interactions phenolic compound-salivary protein, determination of the HPLC-DAD profile of salivary proteins before and after the treatment with phenolic compounds (catechin and epicatechin) has been used. Results showed noticeable enhancement of the interaction between (epi)catechin and proline-rich proteins when both types of proteins are blended (see Fig.1), which might indicate that the presence of acidic proline-rich proteins fraction could stabilize the complex formed between proline-rich proteins and flavanols.
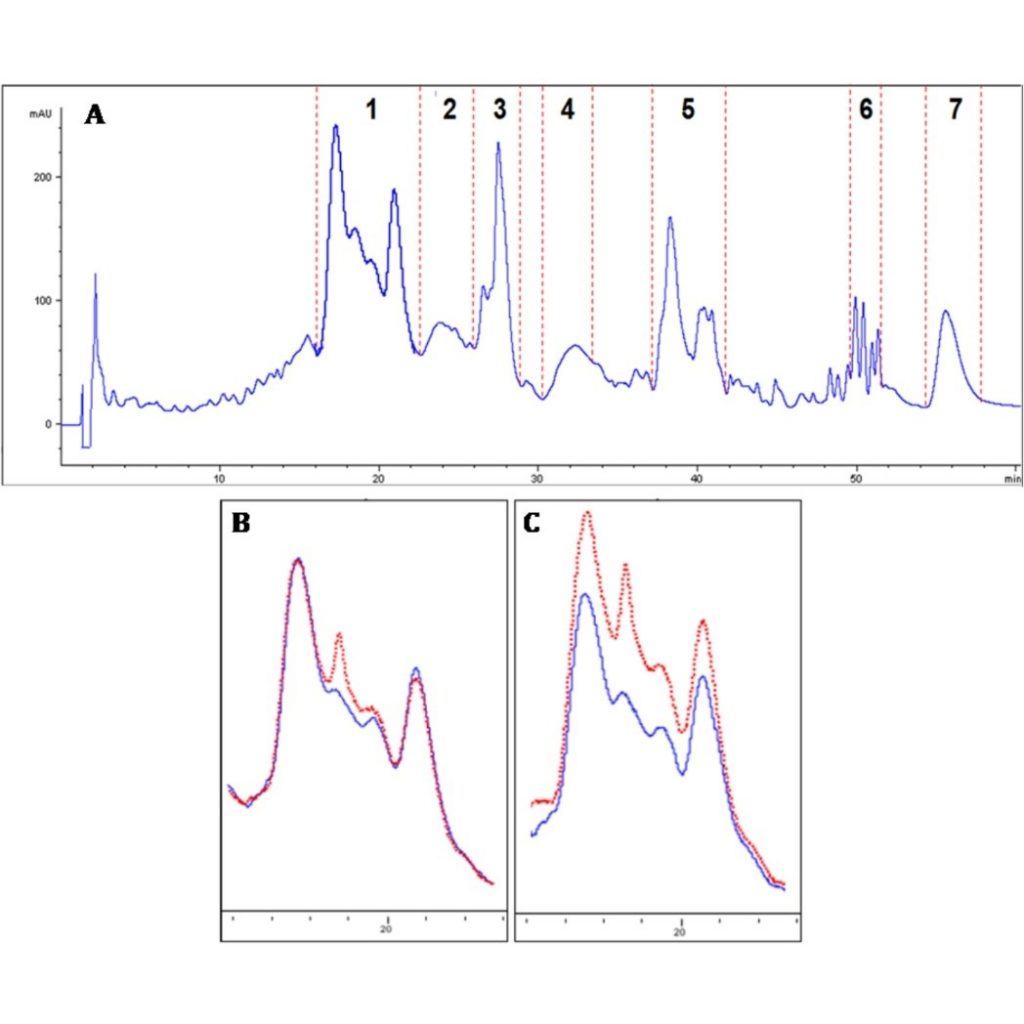

To deepen in the characteristics of the possible soluble aggregates formed, the apparent size of the aggregates was determined by dynamic light scattering (DLS) and the identification of the aggregates formed was performed by MALDI-TOF analyses. Results obtained with the different techniques showed the different behaviour of the salivary proteins fractions when they are isolated compared to when blends of salivary proteins are assayed. Up to 30 soluble aggregates were tentatively identified with molecular weight from 4680 to 35,851. (epi)Catechins seem to bind preferentially proline-rich proteins than acidic proline-rich proteins, although the medium size aggregates flavanol- proline-rich proteins formed could favour the interaction with acidic proline-rich proteins giving rise to soluble mixed aggregates (Ramos-Pineda et al., 2019).
Those interested in a longer length report can download the working paper at:
https://www.sciencedirect.com/science/article/pii/S030881461831416X
Research Group: (Left to right) Alba M. Ramos Pineda, Dr Ignacio García Estévez, Dr Cristina Alcalde Eon, Dr. Montserrat Dueñas Patón, Rebeca Ferreras Charro, Dr Elvira Manjón Pérez, Dr. M. Teresa Escribano Bailón. Food Quality Research Team. Grupo de Investigación en Polifenoles (GIP) – Department of Analytical Chemistry, Nutrition and Food Sciences – University of Salamanca (USAL), Salamanca, Spain. Our research projects are focused on the study of the role of phenolic compounds on quality and stability of food products. The main research topic is related to the impact of the phenolic maturity of red grapes on sensory quality of red wines, namely on color and astringency properties. Recent works aimed to go deepen on the study of the mechanism for astringency development through the use of physical-chemical studies, in order to provide the wine industry (both wineries and oenological industries) with basic knowledge about this complex sensation. Furthermore, our studies are also related to the analysis and characterization of phenolic composition (mainly flavonoids and phenolic acids) of different food and plant matrices. This work was carried out in collaboration with Dr. Susana Soares and Dr. Victor de Freitas from the research group of Applied Organic Chemistry (QUINOA) from the Green Chemistry Laboratory (LAQV/REQUIMTE), Department of Chemistry and Biochemistry, University of Porto, Porto, Portugal.
References:
- ASTM. (2004). Standard Definitions of Terms Relating to Sensory Evaluation of Materials and Products. (American Society for Testing and Materials, Ed.). Philadephia.
- Cala, O., Dufourc, E. J., Fouquet, E., Manigand, C., Laguerre, M., & Pianet, I. (2012). The colloidal state of tannins impacts the nature of their interaction with proteins: the case of salivary proline-rich protein/procyanidins binding. Langmuir, 28, 17410-17418.
- Canon, F., Paté, F., Cheynier, V., Sarni-Manchado, P., Giuliani, A., Pérez, J., Durand, D., & Cabane, B. (2013). Aggregation of the salivary proline-rich protein IB5 in the presence of the tannin EgCG. Langmuir, 29, 1926–1937.
- Canon, F.; Ployon, S.; Mazauric, J. P.; Sarni-Manchado, P.; Réfrégiers, M.; Giuliani, A.; Cheynier, V. Binding site of different tannins on a human salivary proline-rich protein evidenced by dissociative photoionization tandem mass spectrometry. Tetrahedron 2015, 71, 3039–3044.
- Castagnola, M., Cabras, T., Vitali, A., Sanna, M. T., & Messana, I. (2011). Biotechnological implications of the salivary proteome. Trends in Biotechnology, 29, 409–418.
- de Freitas, V., & Mateus, N. (2012). Protein/Polyphenol Interactions: Past and Present Contributions. Mechanisms of Astringency Perception. Current Organic Chemistry, 16, 724–746.
- Ferrer-Gallego, R., Hernández-Hierro, J. M., Brás, N. F., Vale, N., Gomes, P., Mateus, N., de Freitas, V., Heredia, F. J., & Escribano-Bailón, M. T. (2017). Interaction between Wine Phenolic Acids and Salivary Proteins by Saturation-Transfer Difference Nuclear Magnetic Resonance Spectroscopy (STD-NMR) and Molecular Dynamics Simulations. Journal of Agricultural and Food Chemistry, 65, 6434–6441.
- Inzitari, R., Cabras, T., Onnis, G., Olmi, C., Mastinu, A., Sanna, M. T., Pellegrini, M. G., Castagnola, M., & Messana, I. (2005). Different isoforms and post-translational modifications of human salivary acidic proline-rich proteins. Proteomics, 5, 805–815.
- Messana, I., Cabras, T., Inzitari, R., Lupi, A., Zuppi, C., Olmi, C., Fada, M. B., Cordaro, M., Giardina, B., & Castagnola, M. (2004). Characterization of the human salivary basic proline-rich protein complex by a proteomic approach. Journal of Proteome Research, 3, 792–800.
- Oppenheim, F. G., Salih, E., Siqueira, W. L., Zhang, W., & Helmerhorst, E. J. (2007). Salivary proteome and its genetic polymorphisms. Annals of the New York Academy of Sciences, 1098, 22-50.
- Ramos-Pineda, A. M., García-Estévez, I., Soares, S., de Freitas, V., Dueñas, M., Escribano-Bailón, M.T. (2019). Synergistic effect of mixture of two proline-rich-protein salivary families (aPRP and bPRP) on the interaction with wine flavanols. Food Chemistry, 212, 210-215.
- Rinaldi, A., Gambuti, A., & Moio, L. (2012). Precipitation of Salivary Proteins After the Interaction with Wine: The Effect of Ethanol, pH, Fructose, and Mannoproteins. Journal of Food Science, 77, 485–490.
- Soares, S., Vitorino, R., Osório, H., Fernandes, A., Venâncio, A., Mateus, N., Amado, F., De Freitas, V. (2011). Reactivity of human salivary proteins families toward food polyphenols. Journal of Agricultural and Food Chemistry, 59, 5535–5547.

