By Maria del Carmen Portillo
The grape berry surface hosts a microbiota of filamentous fungi, yeast, and bacteria that can have an impact on grape and wine quality (Fleet, 2003; Riberéau-Gayon et al., 2006). When the grape surface is altered (e.g. by damaged skin of the berry, highly compact bunches, excess of humidity, phytopathogen infections) the diversity and the population sizes of the microbiota are affected and can lead to the spoilage of the berry. Grape damage of the harvested bunches and the alteration of the grape ecological balance may compromise the vinification process and the final wine quality typically adding off-flavors (Steel et al., 2013). Thus, it is important to further investigate the microbiota diversity changes in damaged grapes and its influence on the alcoholic fermentation.
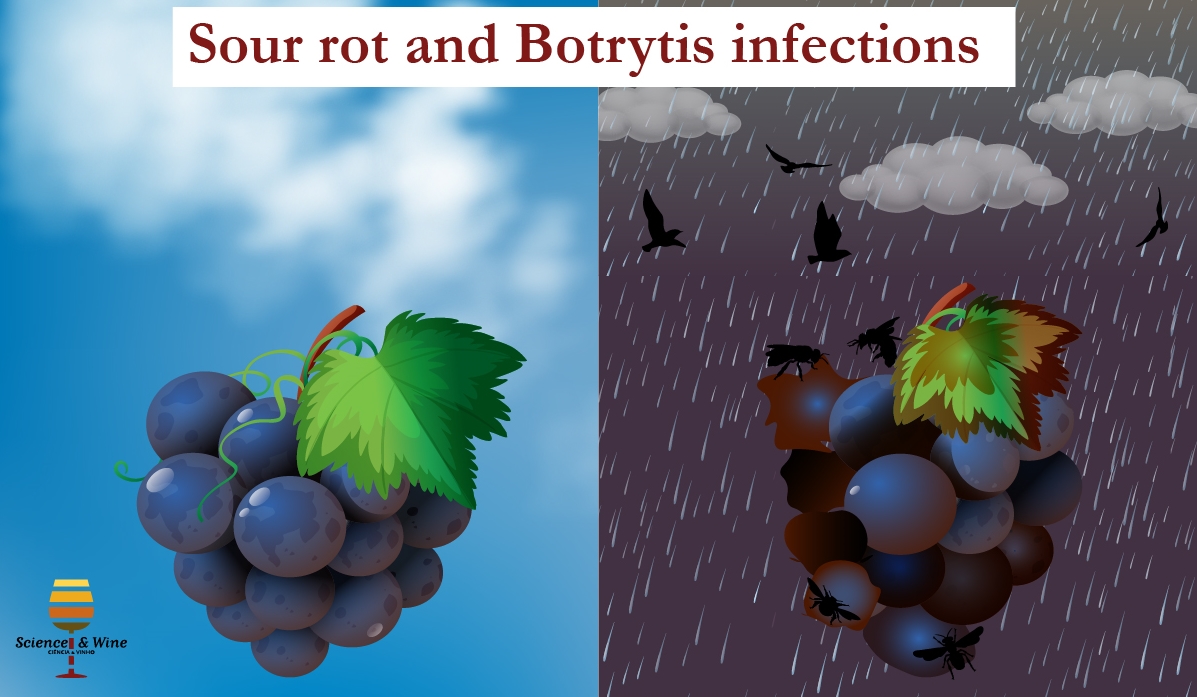
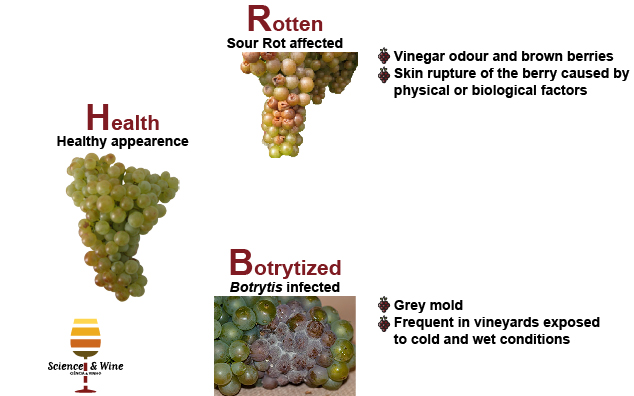
Sour rot and Botrytis infections are the most common causes of heavy grape berry crop losses. The sour rot affects mostly dense bunches close to harvesting and is typically characterized by vinegar odour and brown berries. Disease aetiology is related with the skin rupture of the berry caused by physical factors (e.g. rain, hail, berry abrasion) or biological factors (e.g. insects, birds, moulds). The injuries on grape skin contribute to the development of yeasts and bacteria considered as the main responsible agents of this rot (Huber et al., 2011). Moreover, insects are an important source of microorganisms that can colonize grapes and proliferate once the injury in the skin is done (Barata et al., 2012). Botrytis infection (also known as grey mold) is frequent in vineyards exposed to cold and wet conditions during the ripening period (Nigro et al., 2006). In the case of sweet wines, where the presence of Botrytis cinerea is desired, the grapes are subjected to an extended ripening before harvesting and to a prolonged period of drying before crushing to enhance the abundance of B. cinerea (Stefanini et al., 2016).
Previous studies have documented the microbiota in sound and damaged grapes, including sour rotten and Botrytis-affected grapes (Barata et al., 2012; Mateo et al., 2014; Nisiotou et al., 2011). The results described how grape spoilage affects the grape microbiota, with damaged grapes harboring the highest yeast and acetic acid bacteria (AAB) population (Barata et al., 2008; Mateo et al., 2014). However, most of these studies use culture based techniques probably leading to underestimation of the microbial species involved. Currently, it is accepted that culture-isolated microorganisms are not necessarily representative of the microbial diversity (Amann et al., 1995; Rantsiou et al., 2005). Thus, the reported species selected during grape damaged by sour rot or Botrytis might be biased by the composition of culture media and the capacity of the microbes to grow on them (Cocolin et al., 2000; Millet and Lonvaud-Funel, 2000).
Recently, several culture-independent methods based on the genetic background have been used to analyze the microbial diversity from grapes to wine (reviewed in Cocolin et al., 2011). Generally, the use of molecular biology methods has not only endorsed the traditional results but has also been able to identify higher microbial diversity than previously expected. Despite the potential of molecular techniques, we have just found one work where these were applied to study the microbial diversity of Botrytis-affected grapes (Nisiotou et al., 2011). Specifically, these authors used PCR-DGGE to monitor the yeast population changes during spontaneous fermentations of sound and Botrytis-affected grapes. The results included the detection of some bacterial genera not detected before in sour rot or botrytized musts like Enterobacter, Bacillus and Staphylococcus, some of them capable to survive in fermenting musts (Nisiotou et al., 2011).
Among molecular methods, massive sequencing (MS) technologies are becoming a widely used methodology to characterize more precisely the microbial community of complex environmental ecosystems, including food samples (Ercolini, 2013). For example, MS technologies have allowed metagenomic analysis of vineyard and wine microbiome deciphering which microorganisms are present with higher sensitivity than previous techniques and how their communities are affected by several magnitude factors (reviewed in Morgan et al., 2017).
In our study, we aim to establish the relationship between the sour rot and Botrytis infection affecting Macabeo grapes with specific changes on the grape microbiota. In order to achieve this objective, sound and damaged grapes were harvested and their microbial diversity monitored during subsequent spontaneous alcoholic fermentations by both culture dependent and independent methods including PCR-DGGE, qPCR and MS to weigh the biases introduced by the techniques in an effort to estimate the community changes introduced by sour rot and Botrytis infection.
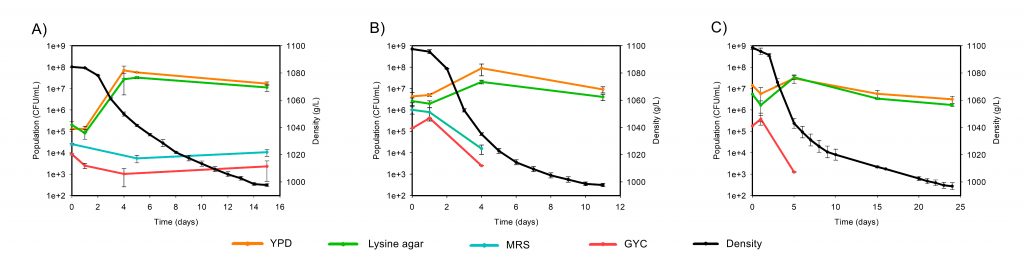
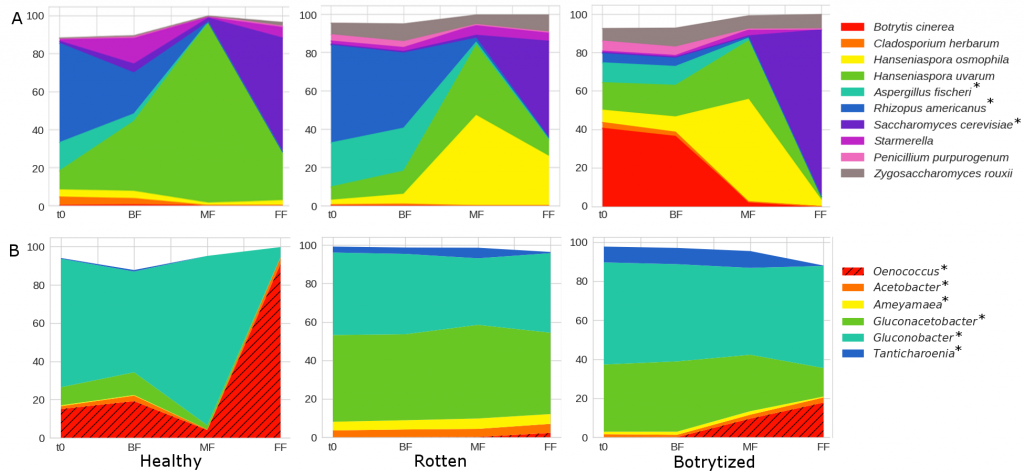
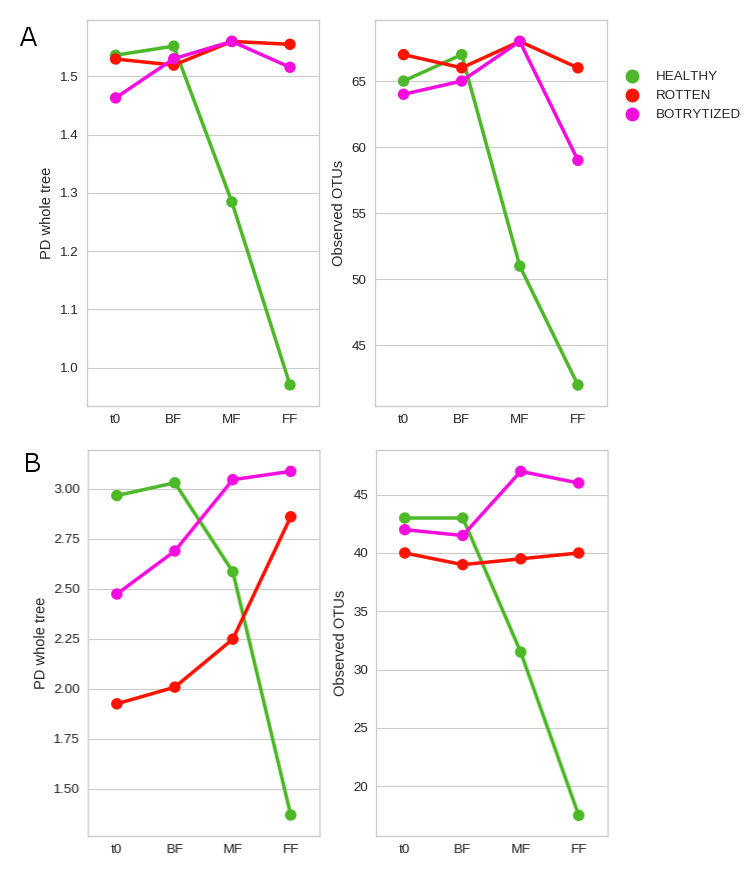
Thus, our study is the first to include the molecular techniques qPCR and MS to evaluate the population evolution along spontaneous fermentation of sour rot and Botrytis affected grapes in comparison with healthy grapes. The results showed that the fermentation proceeded the fastest in the rotten must followed by the healthy and the botrytized grapes. As in previous studies, plate cell counts and qPCR results confirmed the increase in the number of both bacteria and fungi in the musts from damaged grapes. MS detected higher biodiversity than the other techniques at each stage, with Saccharomyces and Oenococcus found already in the grape must. Hanseniaspora osmophila replaced to H. uvarum as the predominant yeast during the mid-fermentation stage for both damaged grapes. Furthermore, musts and beginning of fermentation from rotten and botrytized grapes consistently had a higher presence of the fungi Zygosaccharomyces, Penicillium and Aspergillus while high abundance of Botrytis were observed just for botrytized grapes. As expected, the acetic acid bacteria number increased in musts from rotten and botrytized grapes, mostly due to changes in proportion of the genus of acetic bacteria Gluconoacetobacter which remained more abundant during damaged grapes fermentation than during healthy ones. All this mentioned genera have previously been associated with spoiled fermentations and wines. Interestingly, the presence of the lactic acid bacteria Oenococcus oeni at the end of the alcoholic fermentation was strongly affected by the health status of the grapes. This fact indicates that a good health of the grape is mandatory if a successful and spontaneous malolactic fermentation is desired after the alcoholic fermentation.

Those interested in a longer length report can download the working paper at:
https://www.sciencedirect.com/science/article/pii/S0168160518302757
References
- Amann, R.I., Ludwig, W., Schleifer, K.H., 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59, 143-169.
- Barata, A., Santos, S.C., Malfeito-Ferreira, M., Loureiro, V., 2012. New insights into the ecological interaction between grape berry microorganisms and drosophila flies during the development of sour rot. Microb. Ecol. 64, 416–430.
- Barata, A., Seborro, F., Belloch, C., Malfeito-Ferreira, M., Loureiro, V., 2008. Ascomycetous yeast species recovered from grapes damaged by honeydew and sour rot. J. Appl. Microbiol. 104, 1182-1191.
- Cocolin, L., Bisson, L. F., Mills, D. A., 2000. Direct profiling of the yeast dynamics in wine fermentations. FEMS Microbiol. Lett. 189:81–87.
- Cocolin, L., Campolongo, S., Alessandria, V., Dolci, P., Rantsiou, K., 2011. Culture independent analyses and wine fermentation: an overview of achievements 10 years after first application. Ann. Microbiol. 61, 17-23.
- Ercolini, D., 2013. High-throughput sequencing and metagenomics: moving forward in the culture-independent analysis of food microbial ecololgy. Appl. Environ. Microb. 79, 3148-3155.
- Fleet, H. G., 2003. Yeast interactions and wine flavor. Int. J. Food Microbiol. 86, 11–22.
- González, A., Hierro, N., Poblet, M., Mas, A., Guillamón, J. M., 2005. Application of molecular methods to demonstrate species and strain evolution of acetic acid bacteria population during wine production. Int. J. Food Microbiol. 102, 295-304.
- González, A., Hierro, N., Poblet, M., Mas, A., Guillamón, J. M., 2006. Enumeration and detection of acetic acid bacteria by real-time PCR and nested PCR. FEMS Microbiol. Lett. 254, 123–128.
- Huber, C., McFadden-Smith, W., Inglis, D., 2011. Management and etiology of grape sour rot in the Niagara region. Phytopathology 101, S259–S259.
- Jolly, N.P., Varela, C., Pretorius, I.S., 2014. Not your ordinary yeast: non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 14, 215–237.
- Mateo, E., Torija, M.J., Mas, A., Bartowsky, E. J., 2014. Acetic acid bacteria isolated from grapes of South Australian vineyards. Int. J. Food Microbiol. 178, 98-106.
- Millet, V., Lonvaud-Funel, A., 2000. The viable but non-culturable state of wine microorganisms during storage. Lett. Appl. Microbiol. 30:136–141.
- Morgan, H. H., du Toit, M., Setati, M. E., 2017. The Grapevine and Wine Microbiome: Insights from High-Throughput Amplicon Sequencing. Front. Microbiol. 8, 820.
- Nigro, F., Schena, L., Ligorio, A., Pentimone, I., Ippolito, A., Salerno, M. G., 2006. Control of table grape storage rots by pre-harvest applications of salts. Postharvest Biol. Tec. 42, 142-149.
- Nisiotou, A. A., Rantsiou, K., Iliopoulos, V., Cocolin, L., Nychas, G.J.E., 2011. Bacterial species associated with sound and Botrytis-infected grapes from a Greek vineyard. Int. J. Food Microbiol. 145, 432–436.
- Rantsiou, K., Urso, R., Iacumin, L., Cantoni, C., Cattaneo, P., Comi. G., Cocolin, L., 2005. Culture-Dependent and -Independent Methods To Investigate the microbial ecology of Italian Fermented Sausages. Appl. Environ. Microb. 71, 1977-1986.
- Riberéau-Gayon, P., Dubordieu, D., Donèche, B., Lonvaud, A., 2006. Handbook of Enology. Volume 1. The Microbiology of Wine and Vinifications, second ed. John Wiley & Sons, Ltd, France.
- Steel, C. C., Blackman, J. W., Schimdtke, L. M., 2013. Grapevine Bunch Rots: Impacts on Wine Composition, Quality, and Potential Procedures for the Removal of Wine Faults. J. Agr. Food Chem. 61, 5189−5206.
- Stefanini I., Albanese D., Cavazza A., Franciosi E., De Filippo C., Donati C., Cavalieri, D., 2016. Dynamic changes in microbiota and mycobiota during spontaneous ‘Vino Santo Trentino’ fermentation. Microb. Biotechnol. 9, 195–208