By Elia Romanini and Donato Colangelo
Oxidation of white wine is a complex phenomenon that may occur both in winemaking and during bottle storage. It results in changes in the sensory, color, and aroma attributes (Ferreira, Hogg, & de Pinho, 2003). Oxygen is the limiting factor of this spoilage. Significant amounts of oxygen are stored in the headspace of the bottle, but, in addition, there are two other sources: there are variable amounts of oxygen already dissolved in the wine and some oxygen permeates through the closure. In presence of oxygen, the propensity of the wine to be altered is highly affected by temperature (Godden et al., 2001).
The primary substrates for oxidation in wines are phenolic compounds, especially flavonoids such as flavan-3-ols and their condensed products, proanthocyanidin (Fernandez-Zurbano et al., 1995). In the enological practice, compounds such as sulfur dioxide, ascorbic acid, and glutathione are considered key factors towards wine resistance to oxidation and the preservation of aroma stability (Ugliano et al., 2011).
This work analyzed white wines that were randomly oxidized based on evidence from sensory evaluation and sought to obtain a deeper insight of the oxidative phenomenon through sensory analysis, chemical parameters, and metabolomics. Five different white wines were utilized in this study: Falerno del Massico 2007 DOCG (FAL), Fiano di Avellino 2010 DOCG (FIA), Greco di Tufo 2010 DOCG (GRE), Picolit Colli Orientali del Friuli 2009 DOCG (PIC) and Verduzzo Friulano 2013 (VER). To identify and quantify differences among samples in relation to their oxidation degree, a triangle test (ISO 4120:2004) was carried out on all the wine samples that were tasted the same day.
An untargeted metabolomic screening was performed through high-resolution MS using a QTOF hybrid quadrupole time-flight mass spectrophotometer (Agilent Technologies Santa Clara, CA, USA) coupled with an UHPLC (UHPLC/QTOF-MS) chromatographic system. PCA was used as a descriptive method to examine the relationships among the variables and the grouping among samples (Lewi, 1992). Cross-validation of the OPLS-DA model was carried out to validate the model using CV-ANOVA (p < 0.01), and permutation testing was performed to avoid overfitting. Variable importance in projection (VIP analysis) was used to identify those metabolites with the highest discrimination potential (VIP score > 1.0).
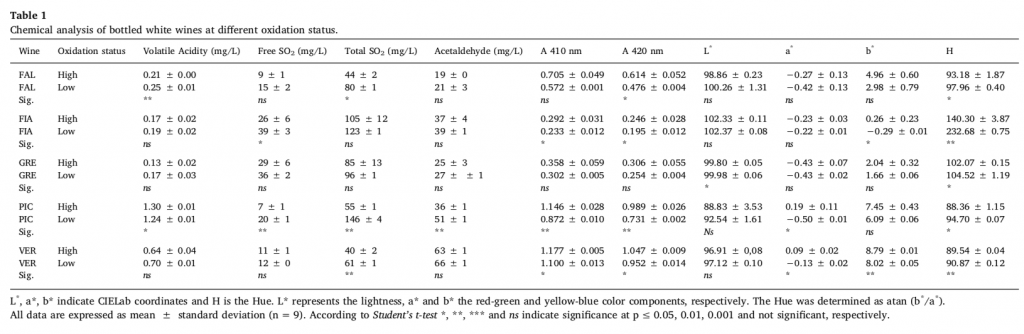
The results from the chemical analyses are shown in Table 1. The wines were categorized into two groups according to their oxidation level as provided by sensory analysis: the score (high or low) was reported as the oxidation status of each sample. In detail, for GRE, FAL, PIC and VER wines, the advanced oxidative status could be deduced by the lower concentrations of total SO2, while significant differences in free SO2 levels between oxidation statuses were observed only for FIA and PIC wines. For both PIC and VER wines, the level of browning (A420nm) and a* were significantly higher in the “high ox” group. Statistics showed Pearson’s negative correlation between free SO2 and A420nm (Figure 1). Consequently, free SO2 levels were higher in samples with a low level of oxidation than in samples with a high level of oxidation.
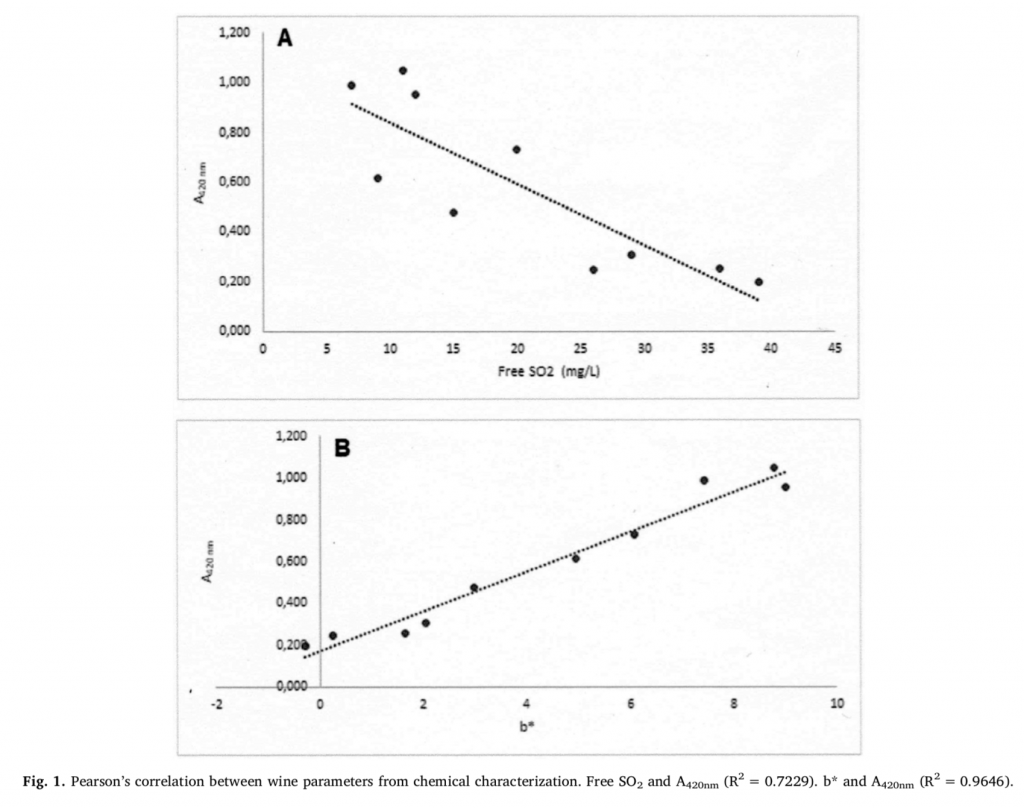
The UHPLC/QTOF-MS metabolomic analysis was elaborated, including the classification of the oxidation status of wines. An unsupervised approach was initially carried out to better highlight the relatedness between the samples. From the fold-change-based cluster analysis (Figure 2), a significant differentiation between the samples could be observed, with the type of wine having a hierarchical priority. Nonetheless, within each type of wine, a separation of the samples based on the level of oxidation was observed. This separation was marked for FAL, PIC, and VER samples, even if FAL samples were not highly discriminated between low and high oxidation status from chemical characterization (Table 1). For GRE and FIA wines, a similar overlap between classes of oxidation has also been outlined for the chemical parameters (Table 1).
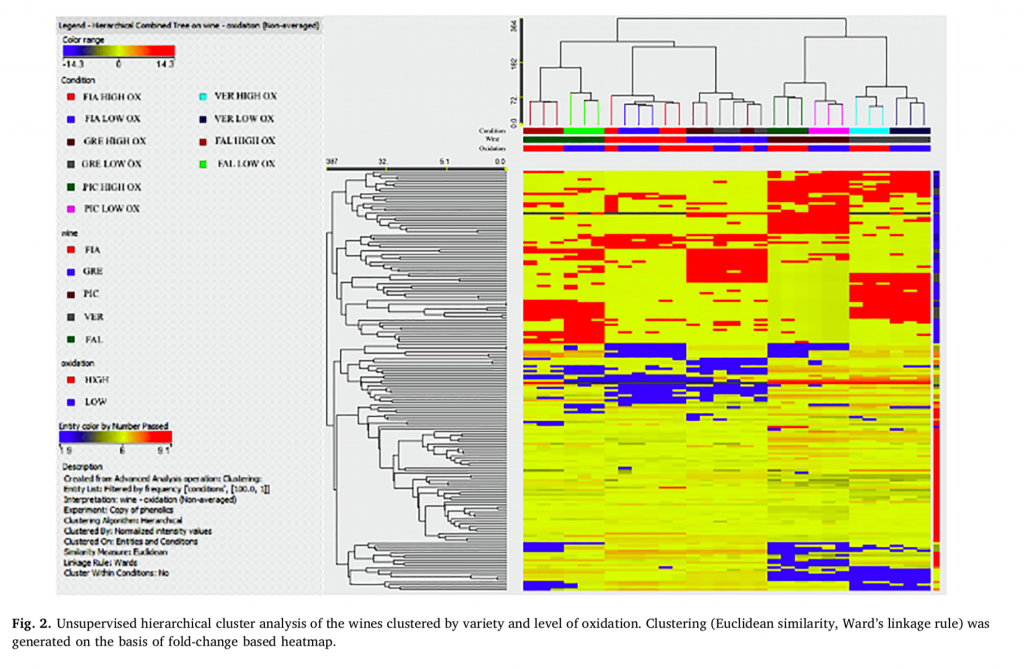
The following supervised OPLS-DA model (Figure 3) provided strong clustering according to the oxidation status. In fact, the goodness-of-fit R2Y was 0.92, and the prediction ability Q2Y was 0.71, both of which were well above the acceptability threshold of 0.5 (Rombouts et al., 2017). In the within factor of the discriminant compounds identified by VIP analysis, molecules included in the classes of anthocyanins, flavanols, flavonols, and phenolic acids were confirmed to be significantly affected when oxidation phenomena occurred in white wine, as already found (Oliveira et al., 2011). In fact, in white wines exposed to oxygen, a significant decrease in total phenols occurs, in which the flavonoid fraction remains stable and only the nonflavonoid fraction decreases (Singleton, 1989).
In our study, lower amounts of phenolic compounds, such as cyanidin 3-O-6′′-p-coumaroyl-glucoside, delphinidin 3-O-glucoside, quercetin 3-O-glucosyl-xyloside, dihydroquercetin, and quercetin 3-O-glucuronide, were observed in the highly oxidized samples. Evidence in the samples with low oxidation status outlined that hydroxycinnamates remain very important for oxidation-related issues in wine browning (Table 1) because they represent the major group of white wine phenols and possess remarkable antioxidant activity and antioxidant capacity (Oliveira et al., 2011).
Flavonols, such as quercetin, myricetin, isorhamnetin, kaempferol and the corresponding flavones, apigenin and luteolin, were highlighted as discriminant compounds of the oxidative status of white wine. 3-Methylcatechol was the only flavanol compound identified to be discriminant in the detection of the oxidative status of the white wines under study. In addition to the VIP scores, a significant difference (p < 0.05) could be observed between the two classes of oxidation for some all the compounds reported, namely delphinidin 3-O-glucoside, cyanidin, poncirin, apigenin 6-C-glucoside, kaempferol, 2-hydroxybenzoic acid.
This study indicated that the content of most of the phenolic compounds identified in the wines was diminished with oxidative aging, with the exception of 6-hydroxyluteolin, 6,8-dihydroxykaempferol, kaempferol 3-O-xylosyl-rutinoside, avenanthramide K, 7-hydroxysecoisolariciresinol caffeic acid, feruloyl tartaric acid, isoferulic acid, and p-coumaric acid ethyl ester.
These discriminant molecules detected as either significantly lower or higher in wines with low or high oxidation levels, respectively, can represent a first step for gaining insights into the complex mechanisms of random oxidation observed in bottled wines.
Those interested in a longer length report can download the working paper at:
https://www.sciencedirect.com/science/article/pii/S0308814619303966

Elia Romanini actually is a PhD student of Agrisystem – Doctoral School on the Agro-Food System. He received his master’s degree in Food Technology from the Università Cattolica del Sacro Cuore (Piacenza) and he worked for 2 years in a craft brewery. He is currently working on sustainability of fermented beverages, in particularly on wine haze forming protein and bentonite alternatives. He spent the last 6 months as a visiting PhD student at Australian Wine Research Institute.

Donato Colangelo is currently a Ph.D. candidate at Università Cattolica del Sacro Cuore in Piacenza, Italy. He received the Master Degree in food technology in 2013. His Ph.D. project has concerned the search for alternative approaches to bentonite fining in white winemaking with a special eye on the drawbacks of each alternative and considerations about the environmental impacts. As a visiting student, in 2017 he collaborated with the University of California, Davis, working on a project about the use and regeneration of cation exchange resins for wine fining aimed at finding winery and environmentally-friendly solutions. He has been working on bentonite effects on wine colloidal stability since 2014. Parallel area of study include the tartaric stability of wines, the effects of the wine matrix factors and of enological adjuvants on the kinetics of tartrate precipitation.
References:
- Fernandez-Zurbano, P.; Ferreira V.; Pena, C.; Escudero, A.; Serrano, F.; Cacho, J., 1995. Prediction of oxidative browning in white wines as a function of their chemical composition. J. Agric. Food Chem. 43 (11), 2813-2817
- Ferreira, A.C.S.; Hogg, T.; de Pinho, P.G., 2003. Identification of key odorants related to the typical aroma of oxidation-spoiled white wines. J. Agric. Food. Chem., 51, 1377-1381.
- Godden, P., Francis, L., Field, J., Gishen, M., Coulter, A., Valente, P., Høj, P. & Robinson, E. 2001. Wine bottle closures: physical characteristics and effect on composition and sensory properties of a Semillon Wine 1. Performance up to 20 months post-bottling. Australian Journal of Grape and Wine Research, 7, 62–105.
- ISO 4120:2004. Sensory analysis – Methodology – Triangle test.
- Lewi P.J., 1992. Multivariate data display. In: “multivariate Pattern recognition in chemometrics”. brereton (Ed.). p.43. Elsevier, London.
- Oliveira, C. M., Ferreira, A. C. S., De Freitas, V., & Silva, A. M. (2011). Oxidation mechanisms occurring in wines. Food Research International, 44(5), 1115–1126.
- Rombouts, C., Hemeryck, L. Y., Van Hecke, T., De Smet, S., De Vos, W. H., & Vanhaecke, L., 2017. Untargeted metabolomics of colonic digests reveals kynurenine pathway metabolites, dityrosine and 3-dehydroxycarnitine as red versus white meat discriminating metabolites. Scientific Reports 7, Article number: 42514.
- Singleton, V.L. 1989. Browning and oxidation of must and wines. In Proceedings 4th Annual Midwest Regional Grape and Wine Conference. D.V. Peterson et al. (Eds.), pp. 87-93. State Fruit Experiment Station, Southwest Missouri State University, Mountain Grove.
- Ugliano, M.; Kwiatkowski, M.; Vidal S. P.; Capone, D.; Siebert, T.; Dieval, J-B.; Aagaard, O, Waters, E.J., 2011. Evolution of 3-mercaptohexanol, hydrogen sulfide and methyl mercaptan during bottle storage of Sauvignon blanc wines. Effect of glutathione, copper, oxygen exposure and closure-derived oxygen, Journal of Agricultural and Food Chemistry, 59(6), 2564-2572.

