By Vittorio Capozzi
The study and the employment of selected autochthonous yeasts in order to start and steer alcoholic fermentation in regional wines is a practice explored due to the aptitude of these biotypes to modulate wine quality. In fact, selected eukaryotic resources might offer solutions i) for the adaptation to specific environmental conditions, ii) for specific technical issues of oenological interest and/or iii) for differentiating organoleptic properties of final products (Martínez-Rodríguez et al., 2001; Torresi et al., 2011; Capozzi et al., 2015; Tofalo et al., 2016). This microbial regimen has been receiving increasing attention in reason of the recent evidences underlining an association of microbial diversity with vineyard environments (Bokulich et al., 2014; Knight et al., 2015), suggesting the existence of a sort of ‘microbial terroir’ to be exploited in oenology (Gilbert et al., 2014).
Sparkling wines contain high amount of CO2 and are the result of re-fermentation of a still wine, usually called base wine. Several ingredients, such as sucrose, selected yeasts, bentonite and some nutrients, are added to base wine in order to induce the re-fermentation. Then wines can be bottled, fermented and aged for a long period (about 9-12 months). The use of autochthonous yeasts as starter cultures for secondary fermentation has been recently suggested to enhance the specific features of typical regional sparkling wines and prevent fermentative problems (Torresi et al., 2011; Garofalo et al., 2016). Starter cultures selected for sparkling wine production must satisfy several characteristics, such as tolerance to high amount of ethanol, low pH, increasing carbon dioxide pressure, autolysis and flocculation.
Following the work by Vigentini et al. (2017), in a recent scientific article (Garofalo et al., 2018), we investigated the use of select autochthonous yeast resources for sparkling wine production. We genetically characterized about 160 Saccharomyces cerevisiae autochthonous strains isolated from “Uva di Troia” (an autochthonous Apulian (Southern Italy) grape variety used to produce “Nero di Troia” wine) spontaneous fermentation, after the evaluation of intraspecific diversity using interdelta analysis, we selected one representative strain for each of the 16 genetic cluster (Figure 1).
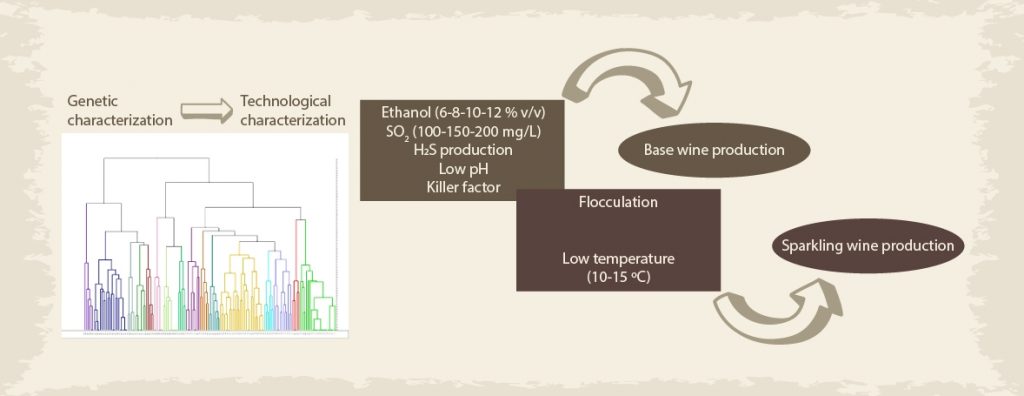
These strains were technological characterized at the lab scale and, successively, tested in winery for both induce alcoholic fermentation in base wine and re-fermentation of sparkling wine (Figures 1 and 2). For re-fermentation trials, yeast fermentative performances were tested in white and rosé sparkling wines obtained from Apulian grape varieties. We reported a complete polyphasic characterization of native yeasts for sparkling wine, highlighting a certain correlation between stress tolerance assays and fermentative aptitude and different performances of the same biotypes in base wine, white and rosé sparkling wines. The study, that for the first time includes VOCs determination in the characterization of autochthonous yeasts strains for during the re-fermentation phase, led to the selection of candidate starter cultures for Apulian sparkling wines.
The research was supported by the Apulian Region Project “Innovazioni di processo e di prodotto nel comparto dei vini spumanti da vitigni autoctoni pugliesi” (IProViSP) and carried out in collaboration with Carmela Garofalo, Carmen Berbegal, Francesco Grieco, Maria Tufariello, Giuseppe Spano, and people from the winery ‘Agricole Alberto Longo’.

Those interested in a longer length report can download the working paper at:
https://www.sciencedirect.com/science/article/pii/S0168160518303453

Vittorio Capozzi is researcher in Food and Agricultural Microbiology at the University of Foggia. His main research interest deals with the study of the complex impact of microorganisms on wine quality and with the understanding of the stressful life of malolactic bacteria in wine environment. Other ongoing research activities focus on the potential application of lactic acid bacteria and yeasts for the design of functional food, antimicrobial activity, flavour enhancements and nutritional improvements. He co-authored more than 100 scientific publications. ORCID: http://orcid.org/0000-0002-0717-0753; Scholar profile: https://scholar.google.it/citations?user=MC3xZQIAAAAJ&hl=it&oi=ao.
References:
- Bokulich, N. A., Thorngate, J. H., Richardson, P. M. & Mills, D. A. (2014). Proceedings of the National Academy of Sciences (PNAS), 111, E139–E148
- Capozzi, V., Garofalo, C., Chiriatti, M. A., Grieco, F. & Spano, G. (2015). Microbiological Research, 181, 75–83
- Garofalo, C., Berbegal, C., Grieco, F., Tufariello, M., Spano, G., & Capozzi, V. (2018). International Journal of Food Microbiology, 285, 7–17
- Garofalo, C., Arena, M.P., Laddomada, B., Cappello, M.S., Bleve, G., Grieco, F., Beneduce, L., Berbegal, C., Spano, G., & Capozzi, V. (2016). Fermentation, 2, 21
- Gilbert, J. A., Lelie, D. van der & Zarraonaindia, I. (2014). Proceedings of the National Academy of Sciences (PNAS), 111, 5–6
- Knight, S., Klaere, S., Fedrizzi, B. & Goddard, M. R. (2015). Scientific Reports, 5, 14233
- Martı́nez-Rodrı́guez, A., Carrascosa, A. V., Barcenilla, J. M., Angeles Pozo-Bayón, M. & Carmen Polo, M. (2001). Food Microbiology, 18, 183–191
- Tofalo, R., Perpetuini, G., Di Gianvito, P., Arfelli, G., Schirone, M., Corsetti, A., & Suzzi, G. (2016). Journal of Applied Microbiology, 120, 1574–1584
- Torresi, S., Frangipane, M. T. & Anelli, G. (2011). Food Chemistry, 129, 1232–1241
- Vigentini, I., Barrera Cardenas, S., Valdetara, F., Faccincani, M., Panont, C.A., Picozzi, C., & Foschino, R. (2017). Frontiers in Microbiology, 8, 1225

