By Susana Ferreyra, Rubén Bottini, Ariel Fontana
This post is a summary of recently published results concerning the investigation of phenolic compounds determination in different winemaking products by tandem absorbance and fluorescence detection following liquid chromatography (LC-DAD-FLD) [1].
Phenolic composition of wines and its by-products such as pomace, bunch stem and cane extracts is of relevance because it is connected with some healthy benefits, including the prevention of chronic diseases such as cancer, obesity, diabetes, or coronary diseases, as well as other biological properties of interest [2-5]. Several studies confirm that the aforementioned health-beneficial properties and bioactivity of wine industry derived products are correlated with the presence of phenolic compounds (PCs). Phenolic composition of wines is also one of the most important quality parameters, contributing to organoleptic properties such as bitterness, astringency, color, flavor, odor and oxidative stability [6]. In addition, the phenolic profile of wines can be a very useful distinctive feature to guarantee, for example, its geographical origin or varietal authentication [7].
Functional foods have acquired an increased awareness of consumers in the last years, highlighting the importance of the determination of chemical characteristics of food products. These data may afford valuable information related to characterization of foods, increasing also their economic and health significance. Thus, the development of analytical methods for determination of PCs in wine industry derived products is a topic of continuous interest.
Liquid chromatography coupled to different detectors such as ultraviolet (UV), fluorescence (FL) and mass spectrometry (MS) have been the choice for analysis of PCs [8, 9]. Although MS is becoming almost mandatory to study the composition of extracts of different nature, some other detection techniques such as UV absorption or FL could be good options. Even though FL, in some cases, has shown considerably higher selectivity and sensitivity, it has not been sufficiently explored as an alternative to UV detection. Besides, its success as a robust and reliable detection technique makes it a viable alternative to MS as well [10]. A related benefit of FL compared to UV detection is its ability to discriminate an analyte from interferences or background peaks. In fact, due to FL detection has different requirements in the structure of the molecule compared to the almost ubiquitous UV detection, a better selectivity and sensitivity could be achieved. A limited quantity of LC-FLD approaches in the field of winemaking industry analysis have been published, and they have been mainly focused on the determination of few compounds belonging to some particular families such as some flavanols (proanthocyanindins) [11, 12] and stilbene derivatives [12, 13] which are those families showing better sensitivities with this detector. Yet other PCs showed better response with DAD detection. These include phenolic acids, flavonols and their derivatives which are abundant in wine industry derived products and which quantification is important for traceability, quality control or industrial application purposes.
Taking into account, we developed and validated a new analytical method for the quantitative determination of the main PCs in wines, bunch stem and grape cane extracts by LC coupled with tandem DAD and FLD detection. The method focused on the simultaneous determination of more than 30 compounds representing different biochemical classes (phenolic acids, flavanols, flavonols, stilbenes, phenylethanol analogues and other compounds).
Based on our previous experience, the separation of analytes was based on a previous chromatographic method reported by our group which separated nearly 20 analytes and chromatograms were recorded by UV at 254, 280, 320 and 370 nm [14]. Considering that the new method was aimed to analyse more than thirty PCs in complex grape derived samples, the possibility of interferences using only UV were increased. Thus, FLD was evaluated as tandem detector to increase the selectivity and sensitivity of the original UV detection for flavanols, stilbenes and phenyl ethanol analogues. In this way and after optimization of FLD conditions, an excitation wavelength of 290 nm was selected and three different emission wavelengths (315, 360 and 400 nm) were recorded according to the elution order of compounds and its sensitivity. Moreover, this mode allowed the quantification of analytes with emissions saturating the detector by using a less sensitive wavelength. This is a plus to determine analytes whose concentration range varies a lot depending on the selected sample (a fact frequently occurring with PCs in different winemaking products) and in other vegetable samples.
The achieved results showed that FLD was the best option for quantifying flavanols, stilbenes and phenyl ethanol analogues families of PCs. In addition, other common analytes in winemaking products such as phenolic acids also produced a residual fluorescence under these conditions, while flavonoids were undetectable by FLD.
The DAD chromatogram of a mix of studied PCs added to samples showed overlapping between some compounds, such as in the case of piceatannol which was incompletely resolved from dihydroquercetin 3-rhamnoside (astilbin). Thus, and taking into account our original aim of combining both detectors, UV detection was not specific for some compounds and multiple components were detected at similar UV absorption wavelengths. When shift to fluorescence detection, the piceatannol signal increased, while signal of astilbin completely disappeared. This result is showed in Fig. 1.
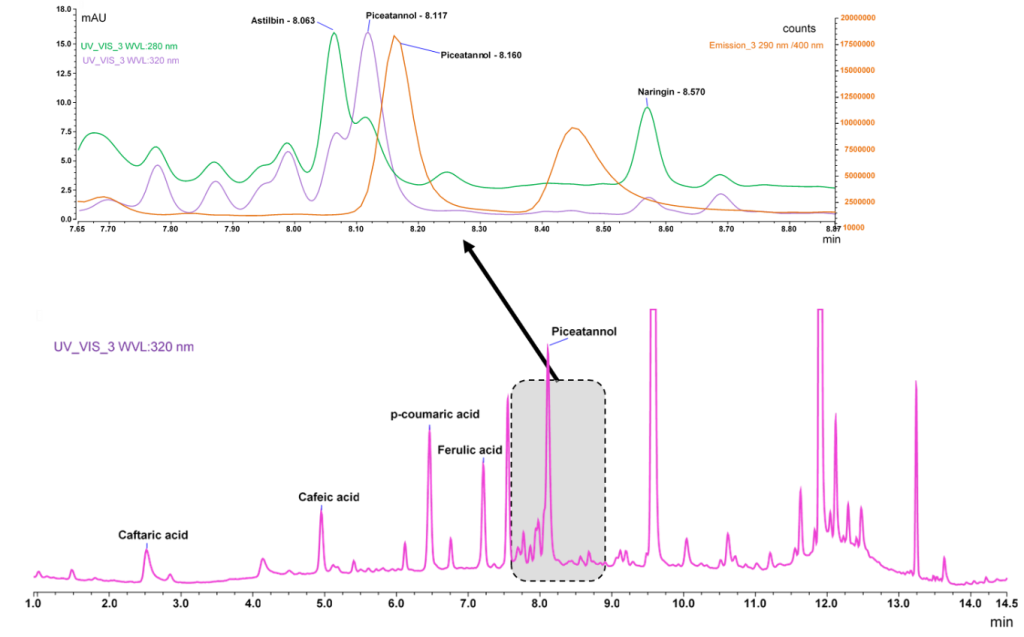
The decreases of signals for interferences or other analytes normally better detected in DAD, and the increase of signal for piceatannol clearly demonstrates the advantages of hyphenating DAD with FLD. The on-line combination of both detectors allowed the selective determination and quantification of these difficult pairs as well the diminution or elimination of matrix effects. Apart from selectivity, the sensitivity was also enhanced for flavanols, stilbenes and phenyl ethanol analogues as is shown in Table 1.
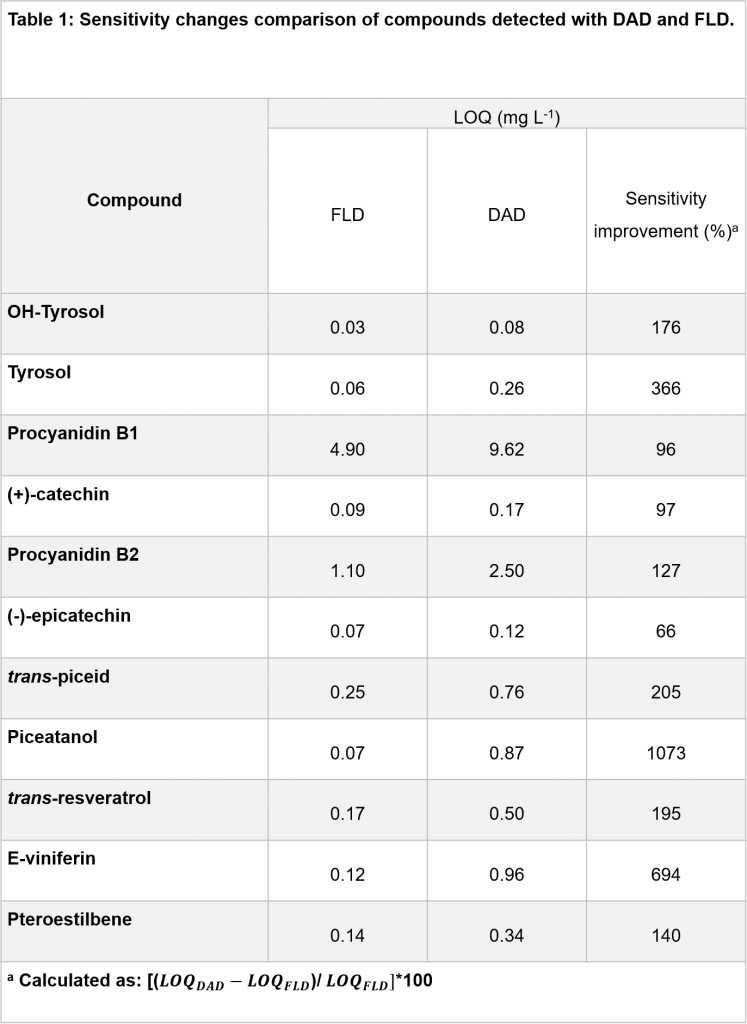
The method was validated by studying the linearity, limits of detection (LOD) and quantification (LOQ), and precision, giving satisfactory results for all analytical parameters. Because the LC was connected in series to DAD and FLD, the method validation was carried out simultaneously, calculating analogue parameters for both detectors in the case of flavanols, stilbenes and phenyl ethanol analogues. As can be observed from Table 1, the combination between DAD and FLD enhanced between 65 to 1000 % the LOQs for flavanols, stilbenes and phenyl ethanol analogues. Fluorescence detection offers much higher sensitivity than UV detection and is a better choice for the detection of these families of compounds. On the other hand, DAD gave better sensitivity for phenolic acids, flavonols and gallate substituted flavanols.
The proposed method as compared with previous works using DAD, FLD or a combination of these detectors for the determination of PCs, showed advantages in terms of the variety of determined compounds. The combination of DAD and FLD allowed to determine differentially those analytes showing best response in each detector. In comparison to other papers using FLD, most of them were focused on the quantification of individual families such as flavanols and stilbenes, or a combination of compounds from both families [10, 12, 13, 15, 16]. The maximum number of compounds that were quantified in these papers was eleven. It is well known that plants produce a lot of PCs also including phenolic acids, flavonols and other derived compounds which are of interest for characterization and could be markers for quality or authentication purposes. Specifically, for winemaking industry derived products there are a lot of evidence related to the importance of PCs profiling for quality control and adding value to commercial products. In this way, expanding the variety of compounds that can be determined with simple, repeatable, reliable, affordable and easily adaptable methodologies is of relevance. The proposed method could be applied to quantify more than 30 PCs in winemaking derived products and other matrices, noticeably plant extracts, without spending much time and expenses.
Finally, the developed method was applied to study the PCs composition of wines, bunch stem and grape cane extracts from different grape varieties. The composition of these samples is presented in detail in Table 4 of the published paper. As a resume, samples of red wines of Malbec, Cabernet Sauvignon and Cabernet Franc were analysed quantifying a total of 19 PCs.
Bunch stem extracts from Chardonnay, Cabernet Franc and Syrah varieties were analysed detecting a total of 15 PCs, with a prevalence of (+)-catechin, caftaric acid, procyanidin B1 and dihydroquercetin 3-rhamnoside (astilbin). For grape cane extracts, a total of 16, 15 and 15 PCs were determined in Malbec, Cabernet Sauvignon and Sauvignon Blanc varieties, respectively. The most abundant family of compounds was stilbene, which represented the 83 % of PCs (for Malbec sample). The abundance order of compounds was piceatannol, ε-viniferin and trans-resveratrol with levels as high as 7517 μg/gDW.
To conclude, we developed a LC-DAD-FLD method for the determination of 32 PCs in winemaking industry derived products. After a careful evaluation of the fluorescence features of analytes, the correct selection of emission/excitation wavelengths and the most appropriate FL mode (multi-emission channels), the sensitivity and selectivity for flavanols, stilbenes and phenyl ethanol analogues were considerably increased in comparison with DAD. The combination of both detectors allowed the simultaneous quantification of different PCs families showing differential sensitivity in each detector. The applicability of the method was verified by the suitable quantification of analytes in complex samples of wine, bunch stem and grape cane extracts. The developed LC-DAD-FLD approach is highly recommended for processing large sets of samples in routine quality control of extracts or during characterization studies.
Acknowledgements: This work was supported by ANPCyT (Grant number: BID PICT 2017-2325), SIIP-UNCuyo and CONICET.
For more information about this study, go to the DOI: 10.1016/j.foodchem.2020.128030

ARIEL FONTANA
Email: afontana@mendoza-conicet.gob.ar
Instituto de Biología Agrícola Mendoza (IBAM) UNCuyo-CONICET,
PhD Chemistry; Chemistry, Biochemistry and Pharmacy Faculty, National University of San Luis, Argentina.
B.A. Biochemistry; Faculty of Biochemistry and Biological Sciences, National University of Litoral, Santa Fe, Argentina. Rate: 8.29/10
Author of a total 45 publications (including 3 book chapters). The h index is 17 with a total citations’ number of 1046. More than 50 presentations in international and national conferences.
Director of a project from the Organization for the Prohibition of Chemical Weapons (OPCW) for peaceful applications of chemistry: “Characterization of bioactive phytochemicals from winemaking by-products of Argentina: exploring stilbenes and lipid-soluble compounds by using metabolomics tools”. Amount: EUR 25.000. Director of the SECTyP-UNCuyo 2018: “Study of bioactive compounds from canes and bunch stems from grapevines: characterization and functionality”.
References
- S. Ferreyra, R. Bottini, A. Fontana, Tandem absorbance and fluorescence detection following liquid chromatography for the profiling of multiclass phenolic compounds in different winemaking products, Food Chemistry, 338 (2021).
- Z. Rasines-Perea, P.L. Teissedre, Grape Polyphenols’ effects in human cardiovascular diseases and diabetes, Molecules, 22 (2017).
- J.M. Lorenzo, M. Pateiro, R. Domínguez, F.J. Barba, P. Putnik, D.B. Kovačević, A. Shpigelman, D. Granato, D. Franco, Berries extracts as natural antioxidants in meat products: A review, Food Research International, 106 (2018) 1095-1104.
- A. Rees, G.F. Dodd, J.P.E. Spencer, The effects of flavonoids on cardiovascular health: A review of human intervention trials and implications for cerebrovascular function, Nutrients, 10 (2018).
- A.R. Fontana, A. Antoniolli, R. Bottini, Grape pomace as a sustainable source of bioactive compounds: Extraction, characterization, and biotechnological applications of phenolics, Journal of Agricultural and Food Chemistry, 61 (2013) 8987-9003.
- M. Atanacković, A. Petrović, S. Jović, L.G. Bukarica, M. Bursać, J. Cvejić, Influence of winemaking techniques on the resveratrol content, total phenolic content and antioxidant potential of red wines, Food Chemistry, 131 (2012) 513-518.
- D.P. Makris, G. Boskou, N.K. Andrikopoulos, Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts, Journal of Food Composition and Analysis, 20 (2007) 125-132.
- M.J. Motilva, A. Serra, A. Macià, Analysis of food polyphenols by ultra high-performance liquid chromatography coupled to mass spectrometry: An overview, Journal of Chromatography A, 1292 (2013) 66-82.
- A. Carrasco-Pancorbo, L. Cerretani, A. Bendini, A. Segura-Carretero, T. Gallina-Toschi, A. Fernández-Gutiérrez, Analytical determination of polyphenols in olive oils, Journal of Separation Science, 28 (2005) 837-858.
- L. Wang, Y. Yamashita, A. Saito, H. Ashida, An analysis method for flavan-3-ols using high performance liquid chromatography coupled with a fluorescence detector, Journal of Food and Drug Analysis, 25 (2017) 478-487.
- B.C. Radovanović, A.N. Radovanović, J.-M. Souquet, Phenolic profile and free radical-scavenging activity of Cabernet Sauvignon wines of different geographical origins from the Balkan region, Journal of the Science of Food and Agriculture, 90 (2010) 2455-2461.
- V. Sáez, C. Gayoso, S. Riquelme, J. Pérez, C. Vergara, C. Mardones, D. von Baer, C18 core-shell column with in-series absorbance and fluorescence detection for simultaneous monitoring of changes in stilbenoid and proanthocyanidin concentrations during grape cane storage, Journal of Chromatography B, 1074-1075 (2018) 70-78.
- X.L. Peng, J. Xu, X.F. Sun, C.J. Ying, L.P. Hao, Analysis of trans-resveratrol and trans-piceid in vegetable foods using high-performance liquid chromatography, International Journal of Food Sciences and Nutrition, 66 (2015) 729-735.
- A.R. Fontana, A. Antoniolli, R. Bottini, Development of a high-performance liquid chromatography method based on a core–shell column approach for the rapid determination of multiclass polyphenols in grape pomaces, Food Chemistry, 192 (2016) 1-8.
- I. Bakhytkyzy, O. Nuñez, J. Saurina, Determination of flavanols by liquid chromatography with fluorescence detection. Application to the characterization of cranberry-based pharmaceuticals through profiling and fingerprinting approaches, Journal of Pharmaceutical and Biomedical Analysis, 156 (2018) 206-213.


[…] This post is a summary of recently published results concerning the investigation of phenolic compounds determination in different winemaking products by tandem absorbance and fluorescence detection following liquid chromatography (LC-DAD-FLD) [1]. […]