This study analysed chemical composition and sensory properties of Amarone wines produced from withered grapes artificially contaminated by Botrytis cinerea and Penicillium spp. Changes in properties of the two wines were evident by comparing wines obtained from healthy grapes used as controls. Penicillium infection affects aroma and sensory profile with respect to wine produced from heathy and botrytized grapes. The differences observed between the two Penicillium wines suggest that the impact on Amarone wine quality may be potentially different depending on the contaminant species of withered grapes. Moreover, strain-species effects cannot be excluded, and it will be possible to assess them in further investigations.
Neuroprotective effect of wine against hydrogen peroxide-induced oxidative stress in human neuron-like cells
Oxidative stress is caused by the insufficient capacity of biological systems to neutralize reactive species produced in excess. A serious imbalance between the generation of reactive oxygen species (ROS) and antioxidant (AOX) protection in favor of the former causes excessive oxidative damage in cells and tissues because the ROS excessive production is associated with disruption of cell cycle regulatory mechanisms. Results obtained suggest that wine is a potential antioxidant and have positive effect against reactive species generated in SH-SY5Y cells, suggesting a neuroprotective effect.
Recent results of an in vitro study reveal that wine compounds have positive effect against reactive species in neuron-like cells, suggesting a neuroprotective effect.
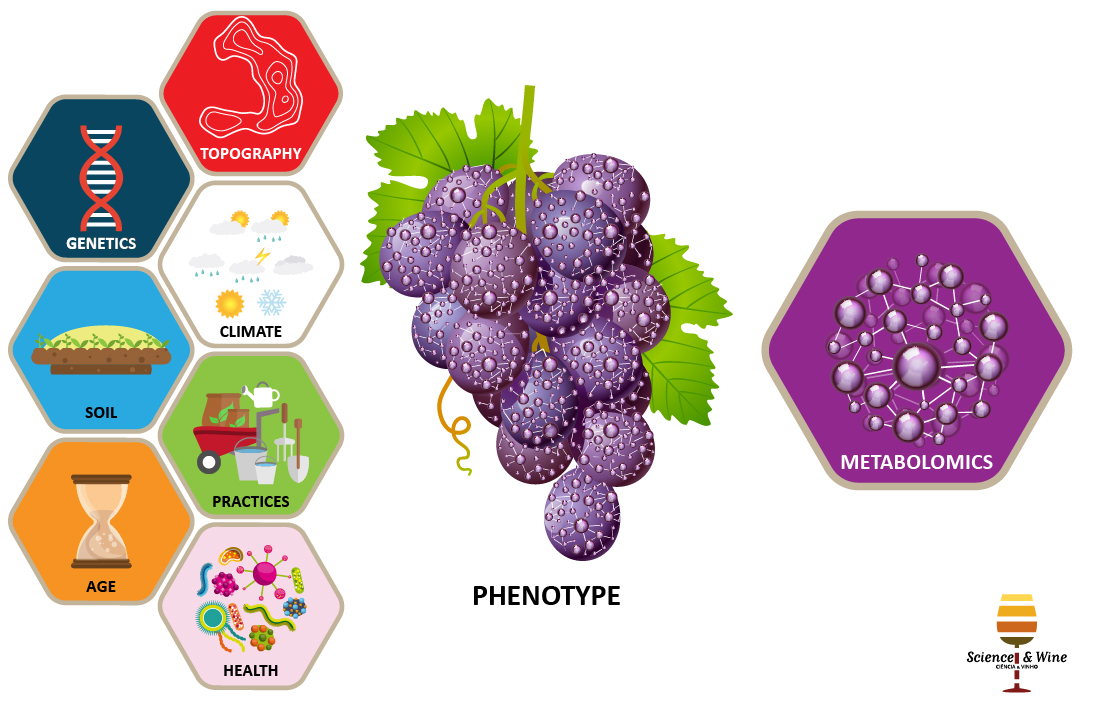
Metabolomics in the field, walking through the chemical diversity of grape
Polyphenols are grape compounds with numerous health benefit and organoleptic properties. These compounds act as key components of the plant defense system against diseases. Herein, are discussed the results of an innovator metabotyping (metabolite-phenotype characterization) study using different grape varieties. A field experiment was setting up with uniform pedo-climatic factors and viticultural practices of growing vines to favor the genetic determinism of polyphenol expression. Metabolite correlation network suggested that several polyphenol subclasses were differently controlled. In a near future, the present polyphenol metabotyping approach coupled to multivariate statistical analyses might assist grape selection programs to improve metabolites with health-benefit potential and plant defense traits.
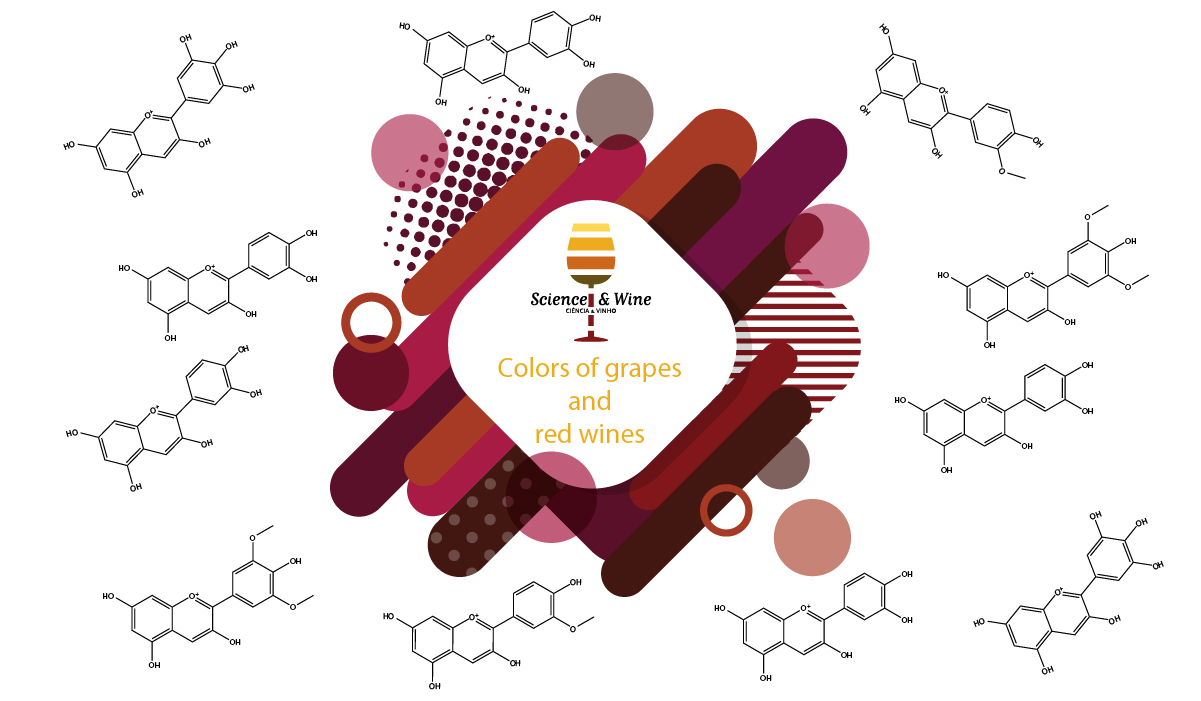
Chemistry and photochemistry inspired by the colors of grapes and red wines
Anthocyanins and pyranoanthocyanins are major contributors to the color of red wines. These pigments are cationic below about pH 3, highly colored, non-toxic, reasonably soluble in water or alcohol and stable to light. They exhibit good antioxidant or antiradical activity and, as part of our diet, confer several important health benefits. excited state proton transfer in uncomplexed anthocyanins or pyranoanthocyanins and ultra-rapid direct deactivation of the excited state in copigmented anthocyanins, contribute to make the color of anthocyanins and pyranoanthocyanins quite resistant to fading in sunlight.